2016 年 22 巻 6 号 p. 817-827
2016 年 22 巻 6 号 p. 817-827
Winter savory extract (WSE) is composed of volatile and non-volatile fractions (WSV and WSN, respectively). We have reported that winter savory volatiles such as WSV, carvacrol or thymol (primary and second components in WSV, respectively) contributed to changes in body surface temperature and core body temperature (BST and CBT, respectively) in humans. In this study, we examined WSN, whose effects on body temperature are unclear, and the mixture of WSN with winter savory volatiles, which likely have a different mechanism of action compared with WSN. WSN ingestion inhibited the decrease in wrist and finger BST, whereas mixtures increased the affected skin parts. Furthermore, the onset of effects at the periphery after mixture ingestion was faster than that after WSN ingestion. These results suggest that volatiles added to WSN greatly influence not only the balance of heat production and transfer, but also the rapid onset of effects at the periphery.
Cold sensitivity or a feeling of cold, known as hie-sho in Japanese, is defined as a chilly sensation in a particular part of the body (Terasawa, 1987). It is thought to be caused by vasoconstriction of peripheral vessels and reduction of skin blood flow (Nagashima et al., 2002; Ushiroyama et al., 2005 and 2006). However, the detailed mechanisms remain unknown.
The ingestion of food providing warmth is one of the effective methods to alleviate cold sensitivity (Nishimura et al., 2012). Winter savory (Satureja montana L.), a herb of the Lamiaceae family, has been used as a food condiment and herbal tea (Başer, 1995; Momtaz and Abdollahi, 2008). Recently, we reported that ingestion of a hot-water extract of dried leaves of winter savory (WSE), which contains a volatile fraction (WSV) and a non-volatile fraction (WSN), inhibited the decrease in body surface temperature (BST) of the wrist, finger and toe in people who experience cold sensitivity (Masuda et al., 2011). In addition, WSV was found to inhibit the decrease in BSTs (wrist, finger and ankle), and the increase in BSTs (forehead and neck) and core body temperature (CBT) (Masuda et al., 2013 and 2016).
On the other hand, as for WSN, according to a preliminary bodily-sensation evaluation, a warm-feeling was induced after ingestion (data not shown). However, the effect of WSN alone on human body temperature is unclear. According to previous reports (Dorman et al., 2004; Ćetković et al., 2007; Gião et al., 2009; Abd El Tawab et al., 2014), the non-volatile fraction in aqueous or alcohol-aqueous extract of winter savory contains a variety of phenolic compounds such as phenolic acids (rosmarinic acid, chlorogenic acid, caffeic acid, ferulic acid, p-coumaric acid, cinnamic acid, gallic acid, syringic acid, protocatechuic acid and vanillic acid) and flavonoids (rutin, quercetin, luteolin, naringenin, (±)-catechin and (−)-epicatechin). Rosmarinic acid, which is found most notably in the Lamiaceae family, is present as a main component in the aqueous or alcohol-aqueous extracts of winter savory. With regard to the vascular function of polyphenolic compounds, the ingestion of polyphenol-rich foods has been reported to contribute to the vasorelaxant and anti-hypertensive effects in animals and humans (Stoclet et al., 2004; Landete, 2012). Moreover, polyphenols such as α-glucosylhesperidin and anthocyanin in black currant extract is reported to improve human body temperature (Takenami et al., 2004; Takumi et al., 2010). Accordingly, WSN may induce a vasorelaxant effect, followed by changes in body temperature.
WSN is mainly composed of hydrophilic compounds, whereas WSV is abundant in lipophilic compounds. In general, the amount of volatiles in the leaves of herbs is extremely small. However, they are of importance because they have a high biomembrane permeability caused by their high lipophilicity (Kohlert et al., 2000; Kato, 2009). As for volatiles, a variety of biological activities have been reported up to the present (Iwai and Nakatani, 1989; Teuscher, 2006; Adorjan et al. 2010; Mikami, 2010). In our study, ingestion of a small dose of volatiles in winter savory [WSV, carvacrol (a main component in WSV), thymol (the second major component in WSV) and the mixture of carvacrol with thymol] contributed to changes in body temperature (Masuda et al., 2013 and 2016). Moreover, as for the onset of this effect, inhibition of the decrease in peripheral BST (wrist and/or finger) was found to occur around 5 or 10 min after ingestion of volatiles in winter savory. Taking into account that, in general, orally administered material is absorbed by the digestive tract and is carried to target organs in the blood stream, the onset of the effects after ingestion of the volatiles in winter savory seems to be rapid. Although the mechanisms of action of WSN and the volatiles in winter savory are unclear, if they are different from one another, the mixture of WSN with the volatiles in winter savory may significantly influence body temperature.
In this study, first, to confirm the vasorelaxant effects of WSN on peripheral vascular smooth muscle, we carried out Exp. 1 using an isolated rat mesenteric artery as a representative resistance vessel. Then, taking into account the results of the in vitro study in Exp. 1, we studied whether WSN (450 mg) ingestion induces changes in human body temperature (in Exp. 2). Next, to elucidate whether the ingestion of a mixture of WSN with the volatiles in winter savory enhances the effects on body temperature, we examined the effects of ingestion of WSE (600 mg, WSE contains WSN and WSV) (in Exp. 3), a mixture of WSN (450 mg) with carvacrol (0.44 mg) (in Exp. 4) or a mixture of WSN (450 mg) with thymol (0.095 mg) (in Exp. 5). The amounts of WSN, WSE, carvacrol and thymol are equivalent to the contents in 2.4 g of dried leaves of winter savory (about one tablespoon).
Preparation of rat mesenteric rings and tension measurements Rat mesenteric arteries were obtained in a similar manner as reported previously (Chino et al., 2013). Mesenteric arteries were immersed in 80 mM KCl Tyrode's solution, which was prepared by replacing the NaCl with an equimolar amount of KCl. The integrity of the endothelium was assessed in all of the preparations by determining the ability of acetylcholine (10 µM) to induce more than 75% relaxation of mesenteric arteries that had been precontracted with noradrenaline (1 µM). The absence of endothelium was confirmed by the lack of relaxation in the presence of acetylcholine (10 µM)
In Exp. 1, each mesenteric artery was pre-contracted with noradrenaline (endothelium-intact ring: 1 µM; endothelium-denuded ring: 0.1 µM). After the noradrenaline-induced contraction reached a steady-state, WSN (100 and 300 µg/mL) was added cumulatively to the bath medium.
Subjects for measurement of BST, CBT and blood flow Clinical tests were conducted with the approval of the Ethics Committee at The University of Shiga Prefecture in accordance with the principles of the Declaration of Helsinki. Informed consent was obtained from all subjects after a full explanation of the content of the consent form.
Subjects were selected using a questionnaire focusing on the symptoms of cold sensitivity as reported by Takumi et al. (2010). The test was carried out in seven, eight, six and eight Japanese female volunteers for Exp. 2, 3, 4 and 5, respectively. Subject characteristics (values are means ± SD) were as follows: in Exp. 2, age, 19 – 22 years; height, 158.0 ± 1.8 cm; body weight, 49.6 ± 4.3 kg; and body mass index (BMI), 19.9 ± 1.4; in Exp. 3, age, 21 – 22 years; height, 159.3 ± 5.8 cm; body weight, 48.0 ± 4.8 kg; and BMI, 18.9 ± 1.1; in Exp. 4, age, 20 – 22 years; height, 156.6 ± 2.2 cm; body weight, 48.4 ± 3.7 kg; and BMI, 19.7 ± 1.7; in Exp. 5, age, 20 – 22 years; height, 154.2 ± 5.3 cm; body weight, 45.8 ± 4.2 kg; and BMI, 19.3 ± 1.1. In order to avoid fluctuations caused by the menstrual cycle, we carried out Exp. 2, 3, 4 and 5 during the luteal phase.
Test protocols for measurement of BST, CBT and blood flow This study was conducted using a randomized, double-blind, placebo-controlled, single-ingestion crossover design in a similar manner as reported previously (Masuda et al., 2011, 2013 and 2016). Exp. 2, 3, 4 and 5 were carried out in late October to late December, in middle December to late January, in early June to late July and in November, respectively. To reduce the influence of seasonal changes in body temperature and to accentuate the differences in body temperature between each sample, room temperatures were set as follows: in Exp 2 and 3, 22 ± 0.5°C; in Exp 4 and 5, 23 ± 0.5°C. Room humidity was maintained at approximately 50% in all experiments.
Samples WSN powder was prepared as follows. Hot water (1800 g, 85°C) was added to the dried leaves of winter savory (60 g, Albania), followed by stirring at 85°C for 10 min. After cooling, the insoluble matter was removed. The filtrate was passed through SEPABEADS® SP70 (Mitsubishi Chemical Co., Ltd., Tokyo, Japan), a synthetic absorbent, followed by elution with 30% aqueous ethanol (1800 g). The eluate was concentrated and lyophilized to obtain the WSN powder (11.5 g, yield against winter savory leaves: 19.2%). The WSE powder (15.3 g, yield against winter savory leaves: 25.5%) was prepared from 60 g of dried leaves of winter savory in 1800 g of hot water (85°C, 10 min) without separation by the synthetic absorbent. Both carvacrol and thymol were obtained as commercially available ingredients (food-additive grade; Sigma Aldrich, St. Louis, MO).
The clinical test was conducted by ingestion of a hard capsule made of gelatin (Matsuya Corporation, Osaka, Japan) containing the sample as follows: in Exp. 2, 450 mg of WSN (total phenol: 132 mg of gallic acid equivalents; rosmarinic acid: 27 mg); in Exp. 3, 600 mg of WSE (total phenol: 132 mg of gallic acid equivalents; rosmarinic acid: 27 mg; carvacrol: 0.44 mg; thymol: 0.095 mg); in Exp. 4, a mixture of WSN (450 mg) with carvacrol (0.44 mg)/corn oil (100 mg, 0.9 kcal, J-Oil Millus, Tokyo, Japan); in Exp. 5, a mixture of WSN (450 mg) with thymol (0.095 mg)/corn oil (100 mg). Placebo capsules in Exp. 2 and 3 contained 600 mg and 450 mg of powdered sugar, respectively. Placebo capsules in Exp. 4 and 5 contained a mixture of 450 mg of powdered sugar and 100 mg of corn oil. Odor was not detected from the capsules. Each capsule was ingested with 37°C water (50 mL).
Measurement of total phenol in WSN and WSE The total phenol content in WSE and WSN was quantified using the Folin–Denis method (Folin and Denis, 1915). Briefly, the reaction mixture containing 2.0 mL of each sample mixed with 2.0 mL of Folin–Denis reagent and 2.0 mL of 10% sodium carbonate was allowed to stand for 1 h at room temperature, and was then measured at 655 nm using a MTP-310Lab absorbance microplate reader (Corona Electric Co., Ltd., Ibaragi, Japan). The total phenol content was expressed as mg of gallic acid equivalent in each sample.
Measurement of rosmarinic acid, carvacrol and thymol in WSN and WSE Quantitative analyses of rosmarinic acid, carvacrol and thymol were carried out using a high-performance liquid chromatography system, as described in a previous report (Masuda et al., 2011). Wavelengths at 310 nm (rosmarinic acid) and 280 nm (carvacrol and thymol) were used for quantification.
Statistical analyses Data are expressed as means ± SEM. In Exp. 1, data were evaluated by one-way analysis of variance (ANOVA) with Tukey's multiple-comparison post-hoc test, using the commercially available software package GraphPad Prism version 5. A probability value of p < 0.05 was regarded as significant. In Exp. 2, 3, 4 and 5, the time-course data were evaluated by two-way repeated measures ANOVA, using GraphPad Prism version 5 (GraphPad Software, Inc., La Jolia, CA). Comparisons between treatment groups at each time point were evaluated using a paired t-test. A probability value of p < 0.05 was regarded as significant.
Vasorelaxant effect of WSN on rat isolated endothelium-intact and endothelium-denuded mesenteric arteries in Exp. 1 In Fig. 1, the vasorelaxant effect of WSN on rat mesenteric arteries (which were precontracted with noradrenaline) is shown to increase in a concentration-dependent manner. In addition, there were no significant differences between the endothelium-intact and the endothelium-denuded arteries.

Vasorelaxant effect of WSN on endothelium-intact and endothelium-denuded isolated rat mesenteric arteries in Exp. 1. Values are expressed as a percentage of the maximal relaxation produced by acetylcholine. The filled columns show relaxation of the endothelium-intact mesenteric artery. The unfilled columns show relaxation of the endothelium-denuded mesenteric artery. Columns show means ± SEM (n = 3 – 4). Values with different superscript letters are significantly different at p < 0.05.
Changes in BST, CBT and blood flow after ingestion of WSN (in Exp. 2), WSE (in Exp. 3), mixture of WSN with carvacrol (in Exp. 4) or mixture of WSN with thymol (in Exp. 5) In Exp. 2, BSTs of the wrist and finger after ingestion of WSN were significantly higher than those after ingestion of the placebo (Fig. 2A and B).

Changes in body temperature at wrist (A), finger (B), ankle (C), forehead (D), neck (E) and tympanic membrane (F) before and after the ingestion of WSN or placebo in Exp. 2.
Values are expressed as means ± SEM. n = 7 (at wrist and finger for −10 to 59 min; p < 0.05 by a two-way repeated measures ANOVA). * indicates p < 0.05 by a paired t-test.
However, other body parts such as ankle, forehead, neck and tympanic membrane [value of tympanic membrane temperature represents CBT (Childs et al., 1999)] did not show significant differences between the WSN- and the placebo-ingestion groups (Fig. 2C – F).
In Exp. 3, there was a significant difference between the WSE- and the placebo-ingestion groups with regard to changes in BSTs (wrist, finger, ankle, forehead and neck) and CBT (Fig. 4A – F).

Changes in body temperature at wrist (A), finger (B), ankle (C), forehead (D), neck (E) and tympanic membrane (F) before and after the ingestion of WSE or placebo in Exp. 3.
Values are expressed as means ± SEM. n = 8 (at wrist, finger, ankle, forehead and neck for −10 to 59 min, at tympanic membrane for −5 to 55 min; p < 0.05 by a two-way repeated measures ANOVA). * indicates p < 0.05 by a paired t-test.
In Exp. 4, changes in BSTs (wrist, finger, ankle and forehead) between the mixture of WSN with carvacrol- and the placebo-ingestion groups were significantly different (Fig. 6A – D). CBT after ingestion of the mixture was significantly higher than that after ingestion of the placebo (Fig. 6F).
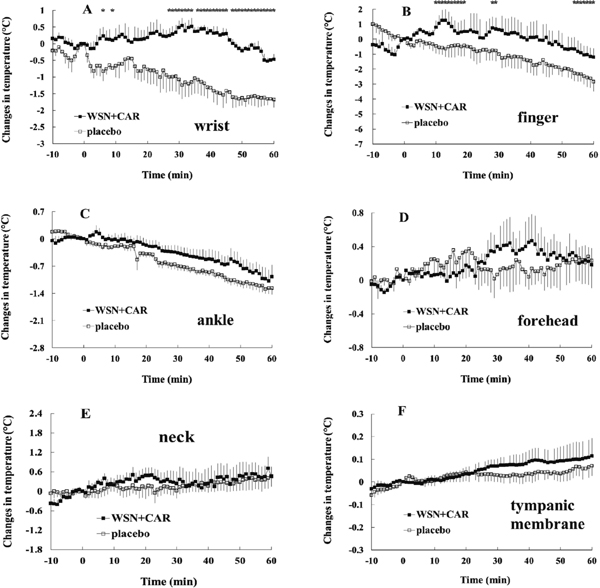
Changes in body temperature at wrist (A), finger (B), ankle (C), forehead (D), neck (E) and tympanic membrane (F) before and after the ingestion of a mixture (WSN and carvacrol: CAR) or placebo in Exp. 4.
Values are expressed as means ± SEM. n = 6 (at wrist and ankle for −10 to 60 min, at finger for −10 to 49 min, at forehead for −10 to 50 min, at tympanic membrane for 0 to 49 min; p < 0.05 by a two-way repeated measures ANOVA). * indicates p < 0.05 by a paired t-test.
In Exp. 5, there was a significant difference between the mixture of WSN with thymol- and the placebo-ingestion groups in changes in BSTs (wrist, finger, ankle, forehead and neck) (Fig. 8A – E). CBT after ingestion of the mixture showed significantly lower than that after ingestion of the placebo (Fig. 8F).

Changes in body temperature at wrist (A), finger (B), ankle (C), forehead (D), neck (E) and tympanic membrane (F) before and after the ingestion of a mixture (WSN and thymol: THY) or placebo in Exp. 5.
Values are expressed as means ± SEM. n = 8 (at wrist, neck and tympanic membrane for −10 to 60 min, at finger and ankle for 0 to 60 min, at forehead for 0 to 50 min; p < 0.05 by a two-way repeated measures ANOVA). * indicates p < 0.05 by a paired t-test.
As for the changes in blood flow of the finger, the ingestion of WSN, WSE or the mixture with thymol provided significantly greater value when compared with the ingestion of the placebo (Fig. 3, 5 or 9, respectively).

Changes in blood flow of the finger before and after the ingestion of WSN or placebo in Exp. 2.
Values are expressed as means ± SEM. n = 7 (for −10 to 59 min; p < 0.05 by a two-way repeated measures ANOVA). * indicates p < 0.05 by a paired t-test.

Changes in blood flow of the finger before and after the ingestion of WSE or placebo in Exp. 3.
Values are expressed as means ± SEM. n = 8 (for −10 to 59 min; p < 0.05 by a two-way repeated measures ANOVA). * indicates p < 0.05 by a paired t-test.

Changes in blood flow of the finger before and after the ingestion of a mixture (WSN and thymol: THY) or placebo in Exp. 5.
Values are expressed as means ± SEM. n = 8 (for 0 to 60 min; p < 0.05 by a two-way repeated measures ANOVA). * indicates p < 0.05 by a paired t-test.
Onset of the treatment effects on BST after ingestion of WSN (in Exp. 2), WSE (in Exp. 3), mixture of WSN with carvacrol (in Exp. 4) or mixture of WSN with thymol (in Exp. 5) To evaluate the onset of treatment effects on body temperature, significant differences between each ingestion group at each time point were evaluated using a paired t-test. As for the inhibition of the decrease in BSTs (wrist and finger), the onset of the effect was as follows: around 30 or 40 min after ingestion of WSN (Fig. 2A and B in Exp. 2); around 5 or 10 min after ingestion of WSE (Fig. 4A and B in Exp. 3); around 5 or 10 after ingestion of the mixture of WSN with carvacrol (Fig. 6A and B in Exp. 4); and around 15 min after ingestion of the mixture of WSN with thymol (Fig. 8A and B in Exp. 5).

Changes in blood flow of the finger before and after the ingestion of a mixture (WSN and carvacrol: CAR) or placebo in Exp. 4. Values are expressed as means ± SEM. n = 6. * indicates p < 0.05 by a paired t-test.
Sasaki et al. (2010) reported that in vitro vasorelaxation studies using isolated mesenteric artery from rats (representatively small resistance vessel) was useful for evaluating herbal medicines for oketsu (state in which blood is static and does not flow smoothly) in humans. Furthermore, the obstruction of blood circulation during oketsu is thought to occur in peripheral blood vessels; therefore, the mesenteric artery has been reported to be more useful than the aorta (larger conductance vessel). Taking into account that cold sensitivity is mostly caused by reductions in peripheral blood flow, studies seeking whether WSN induces vasorelaxant effects are considered valuable. According to the data from Exp. 1, WSN appears to directly induce the relaxation of peripheral vascular smooth muscle. In addition, the vasorelaxant effect was found to be endothelium-independent, and therefore, vasoactive substances produced in endothelial cells such as nitric oxide seem not to be involved in this vasorelaxation. However, further studies elucidating whether the metabolites produced after the absorption of WSN induce the vasorelaxant effect are needed.
As a consequence of the results of Exp. 1, we carried out clinical tests with WSN. In Exp. 2, the ingestion of WSN was confirmed to contribute to the inhibition of the decrease in BSTs of the wrist and finger, but it did not contribute to the changes in BSTs of other body parts (ankle, forehead and neck).
On the other hand, ingestion of WSE (which contains WSN and WSV) increased the number of body parts affected by changes in BSTs (wrist, finger, ankle, forehead and neck, in Exp. 3). In addition, as for CBT, although ingestion of WSN produced no significant increase (in Exp. 2), ingestion of WSE provided a significant increase (in Exp. 3). Comparing the affected areas (in which changes in body temperature are observed) between the WSN- (in Exp. 2) and the WSE- (which contains WSN and WSV, in Exp. 3) ingestion groups, WSV appears to greatly contribute to heat production and heat transfer (followed by changes in CBT and BSTs, respectively) when compared with WSN.
Next, taking into account that addition effects of WSV to WSN, to confirm how each component in WSV contributes to changes in body temperature, we examined the mixture of WSN with carvacrol (primary component in WSV, in Exp. 4) or thymol (second major component in WSV, in Exp. 5). The number of affected skin parts (wrist, finger, ankle, forehead and/or neck) after the ingestion of the mixture of WSN with carvacrol or thymol increased (in Exp. 4 and 5) when compared with the number of those after ingestion of WSN (wrist and finger, in Exp. 2). In addition, CBT increased after ingestion of the mixture of WSN with carvacrol (in Exp. 4). We have reported that carvacrol plays an important role in heat production in the body core (Masuda et al., 2013 and 2016). On the other hand, thymol may greatly contribute to heat transfer from the body core to the body surface without heat production (Masuda et al., 2016). Taking into account that body temperature is dependent on the balance between heat production and heat transfer (Houdas and Ring, 1982; Charkoudian, 2003 and 2010; Guyton and Hall, 2006), we may surmise that after heat production by carvacrol, greater heat transfer occurred from the body core to the body surface, followed by an increase in the number of influenced body parts (in which the changes in BST are observed). On the other hand, as thymol added to WSN was able to facilitate heat transfer from the body core to the body surface without heat production, a decrease in CBT could be induced. Thus, carvacrol or thymol added to WSN could greatly induce changes in the heat balance and body temperature distribution.
With regard to inhibition of the decrease in BSTs (wrist and finger), the onset of the effects by ingestion of WSE (around 5 or 10 min after ingestion, in Exp. 3), the mixture of WSN with carvacrol (around 5 or 10 min after ingestion, in Exp. 4), or the mixture of WSN with thymol (around 15 min after ingestion, in Exp. 5) was faster than that by the ingestion of WSN alone (around 30 or 40 min after ingestion, in Exp. 2).
In general, orally administered material produces pharmacological effects via a mechanism whereby active principles (dissolved in blood directly) interact with target organs. In a single administration, in general, the smaller the Tmax (time becoming the maximal plasma concentration of active principles) is, the faster the onset is (Shigeno, 2015; Kato, 2016). Rosmarinic acid is the main phenolic component in WSN. Rosmarinic acid has been reported to exert vasorelaxant effects using rat isolated thoracic aorta (Ersoy et al., 2008); however, the effects on human body temperature are unclear. As for phenolic compounds showing vasorelaxing effects, recovery effects on peripheral blood flow and body temperature under cold temperature stress have been reported (Takenami et al., 2004; Takumi et al., 2012). Accordingly, a similar effect of rosmarinic acid on human body temperature is estimated. As for the Tmax of rosmarinic acid in human plasma, the value is reported to be 30 min after ingestion (Baba et al., 2005). Moreover, Tmax values of a variety of phenolic compounds such as phenolic acids and flavonoids in human plasma were reported to be more than 30 min after ingestion (Hollman, 2004; Manach et al., 2005). Thus, taking into account the onset of effects by ingestion of WSN (around 30 or 40 min after ingestion, in Exp. 2), we surmise that WSN could induce direct action on peripheral sites via gastrointestinal absorption and the following dissolution in blood. However, the active principles in WSN that contribute to changes in human body temperature remain unclear. Therefore, further investigations to elucidate the active principle in WSN, its bioavailability and pharmacokinetics are needed.
On the other hand, as for thymol, Kohlert et al. (2002) reported that the value of Tmax in human plasma was around 2 h after single administration of thyme extract (which contains thymol as the main volatile component). In addition, carvacrol and thymol have been reported to have similar values of Tmax (Michiels et al., 2008). Taking into account the rapid onset of effects by ingestion of the mixture of WSN with carvacrol or thymol (around 5 or 10 min, or 15 min after ingestion, respectively), an action mechanism that is different from the pathway via circulating blood could be involved in body temperature changes.
Transient receptor potential (TRP) channels, which are non-selective cation channels present in perivascular sensory nerves, have a variety of physiological functions (Holzer, 2011). As for vascular function, the activation of TRP vanilloid 1 (TRPV1) or TRP ankyrin 1 (TRPA1) is thought to induce not only heat production via the central nervous system (CNS, which is greatly related to body-temperature regulation) (Watanabe et al., 1988; Caterina, 2007; Iwasaki et al., 2008) but also the vasorelaxation of peripheral arteries via the release of calcitonine gene-related peptide (CGRP), a potent vasodilator neuropeptide (Caterina, 2007; Earley, 2012; Zygmunt and Högestätt, 2014). Kawasaki and Takahashi (1993) suggested that the release of CGRP and the following inhibition of adrenergic nerve-mediated vasoconstriction could be regulated by CNS. Moreover, as for CGRP, it has been reported that Raynaud's phenomenon, a peripheral circulation disorder, involves a decrease in CGRP-containing perivascular nerves in finger skin (Bunker et al., 1996).
We have confirmed that the volatiles in winter savory (WSV, carvacrol and thymol) can activate TRPA1 (WSV and carvacrol, Masuda et al., 2013; thymol, data not shown) using human TRPA1-expressing cells. According to the report by Ohnuki et al. (2001) and Hachiya et al. (2007), the rapid onset of human body temperature at periphery (around 5 – 10 min after ingestion) was found after the ingestion of CH-19 sweet (non-pungent type of red pepper). Activation of gastrointestinal TRPV1 by capsiate (active principle in CH-19 sweet) is reported to be involved in body temperature changes (Kawabata et al., 2009). Ono et al. (2011) reported that intragastric administration of capsiate in rats induced the stimulation of gastrointestinal TRPV1 and the activation of gastrointestinal vagus nerve, followed by heat production. As for the inhibition of the decrease in peripheral BST (in Exp. 3, 4 and 5), we surmise that the volatiles in winter savory (WSV, carvacrol or thymol) added to WSN could induce rapid responses by a signal transduction mechanism via CNS. However, studies elucidating whether neuronal control via CNS and the release of vasodilator neurotransmitters such as CGRP could be involved in the acceleration of the response are needed. Moreover, the reason that carvacrol and thymol cause different effects (heat production and heat transfer, respectively) is unclear. Therefore, further studies such as investigating absorption, distribution, metabolism and excretion after ingestion of carvacrol or thymol are needed.
In conclusion, we clarified that the ingestion of WSN (the non-volatile fraction in winter savory) inhibited the decrease in BSTs of the wrist and finger only. On the other hand, we demonstrated that the volatiles in winter savory [WSV (volatile fraction in winter savory), carvacrol (main component in WSV) or thymol (second major component in WSV)] added to WSN contributed not only to increases in the affected skin parts (in which changes in body temperature are observed) but also to the fast onset of effects on peripheral BST (wrist and finger) when compared with WSN. We surmise that WSN influences peripheral BST via a non-neural mechanism, whereas the volatiles in winter savory may induce the rapid onset of the effects at the periphery via a neural mechanism.
Acknowledgments We are grateful to Ms. Satsuki Inagaki and Dr. Shu Kaneko of Ogawa & Company, Ltd. for their technical assistance, and to Prof. Tomonori Nadamoto of Koshien University for his helpful advice.