2016 Volume 22 Issue 6 Pages 829-840
2016 Volume 22 Issue 6 Pages 829-840
Effect of hot water extraction on composition and antioxidant activity of prodelphinidins (PDs) from two cultivar bayberry leaves (Biqi and Dongkui) were studied. Composition of PDs were analyzed by NPHPLC-DAD/UV-ESIMS. Dimers to tetramers were separated and each polymers consisted of more than one compounds. Oligomer contents in Biqi extract were considerable, just like high polymers in Dongkui extract. Most dimers (∼4 mg/g) and trimers (∼2.2 mg/g) of Biqi was extracted at 75°C while most tetramers (∼1.7 mg/g) and other polymers (∼11 mg/g) was extracted at 100°C. The highest content of dimers (∼4 mg/g) in Dongkui existed at 50°C. Other polymers showed highest content nearly at 100°C. Antioxidant results (DPPH and FRAP) proved that hot water extract possessed antioxidant activity. In a word, composition of PDs were affected by hot water extraction. Proper extraction condition and materials should be chosen according to your purpose.
In recent years, more and more attention had been paid to proanthocyanidins (PAs) because of their famous pharmacological effects such as antioxidant, antitumor activity and cardiovascular protection ability (Kruger et al., 2014; Oldoni et al., 2016; Way et al., 2015). Prodelphinidins (PDs) belong to PAs, which also include procyanidins, propelargonidins, etc.. Subunits of the three proanthocyanidins are (epi)gallocatechin ((E)GC), (epi)catechin, and (epi)afzelechin, respectively. Structure of PAs are very complex because they have a high structural diversity. They have more than twelve subunits, different of interflavanoid bond, various lengths of chains, and different substitutional groups. Therefore, structural analysis of PAs are difficult and PAs standards are lacking. In order to conduct qualitative and quantitative analysis of PAs, high performance liquid chromatography (HPLC) combined with mass spectra (MS), a commonly and powerful method, was usually employed. Reversed phase HPLC (RPHPLC) can only separate PAs oligomers while normal phase HPLC (NPHPLC) can separate PAs with degree of polymerization up to 12 (Neilson et al., 2016). Silica, diol, and other packing materials are usually used as stationary phase in NPHPLC (Fu et al., 2014; Kuhnert et al., 2015). Mobile phase are low polarity organic solvent (such as dichloromethane and acetonitrile) and little organic acid (Hömmer and Schreier, 2008; Verardo et al., 2015). In MS, negative ESI mode is commonly better than positive mode for PAs because the negative mode shows more sensitivity (Fu et al., 2014).
Trees of bayberry (Myrica rubra Sieb. et Zucc.) which belongs to Myricaceae family were mainly cultivated in southern China for more than 2000 years. They are also native to Japan and other countries of eastern Asia (Chen et al., 2004). In China, local people traditionally cooked a warm concoction using bayberry leaves, twigs, and bark. They consumed bayberry because it was beneficial to human health and had effect on intestinal disorders, ulcers, sores, etc. (Matsuda et al., 2002). Recent researches reported that extracts of bayberry fruits, leaves, and bark had functional activities such as antioxidant, antimicrobial, and antiviral activities (Chen and Liu, 1999; Shen et al., 2004; Xia et al., 2004). It was found that bayberry was rich in polyphenols including phenolic acids, flavones, and flavan-3-ols (Fang et al., 2007; Yang et al., 2011 a). PAs were special polyphenols in bayberry leaves and bark. They were completely PDs type and comprised epigallocatechin-3-O-gallate (EGCG) and few EGC. These features of bayberry leaf PAs were unusual in the plant kingdom because PAs of most plants were procyanidins contained more than one kind of subunits or mixtures of procyanidins, PDs, and propelargonidins (Gu et al., 2003).
Although the infusion of bayberry leaf were consumed by local people for a long time, the components and their change during the cooking processing were not clear. Therefore, the present work focused on PDs composition and their antioxidant activity in hot water extracts. Results would be useful for choosing proper materials and extraction condition and also helpful for developing bayberry leaf as tea.
Regents Folin-Ciocalteu phenol reagent, (+)-catechin, phloroglucinol, 2, 4, 6-tris (2-pyridyl)-s-triazine (TPTZ), and 2, 2-diphenyl-1-picrylhydrazyl radical (DPPH) were purchased from Sigma (St. Louis, Missouri, USA). Sephadex LH-20 was purchased from GE Healthcare Bio-Sciences AB (Sweden). Methanol and dichlormethane for HPLC analysis were HPLC grade. All other organic solvents and chemicals used in this study were of analytical grade commercially available.
Preparation of materials Two cultivars of bayberry leaf (Biqi and Dongkui) were selected for study based on popularity and availability. Leaves were harvested by hand from Xianju in Zhejiang province in south-eastern China. Immature, intermediate, and mature leaves of each cultivar were collected. Immature leaves are bright green while mature leaves are dark green. Color of intermediate leaves is deeper than that of immature leaves but lighter than that of mature leaves. Leaves collected were transported to our lab as soon as possible. They were washed, dried under vacuum at 40°C for about 12 h, ground fine, and stored at −20°C for further experiments.
Extraction of polyphenols and PDs Acetone-water extraction. The prepared leaf powder (1 g) above was extracted with acetone-water (70/30, v/v, 10 mL) for 15 min at room temperature. The extraction process was repeated three times and solution of each extraction were recovered. Acetone was removed by using rotary evaporator under vacuum at 40°C. The remanent aqueous phase was diluted to 1000 mL with methanol. Each sample was prepared in triplicate.
Hot water extraction. The grounded powder (1 g) was added into water (40 mL) in a conical flask with stopper (100 mL). The conical flasks with solution were immersed in a thermostatic water bath for some time. The extraction solution was recovered by filtering. The progress was repeated twice. Some water was added to make a final volume of 100 mL. For determination of mean degree of polymerization (mDP), the extraction solution was not diluted but was lyophilized.
Experimental design. Effects of main factors of water extraction on polyphenols and PDs were tested by single-factor experiment design. Water temperatures (25°C, 50°C, 75°C, 100°C (boiling water)) and extraction time (10 min, 20 min, 30 min) were examined for their effect on content and composition of PDs.
Determination of polyphenols, PDs and hot water extracts Determination of polyphenols. Polyphenols were determined by Folin-Ciocalteu method reported by Xu et al. (Xu et al., 2008) with little modification. Sample solution (1 mL) was mixed with 9 mL distilled water, and 0.5 mL of Folin-Ciocalteu phenol reagent. The mixture was shaken vigorously for 5 min. Then 5 mL of Na2CO3 solution was added. The mixture was immediately diluted to 25 mL with distilled water. After being incubated at room temperature for sixty min, the absorbance of the mixture was recorded at 75 nm. Same volume of distilled-deionized water was used as a blank sample. A calibration curve was prepared by using gallic acid with different concentration and the results obtained were expressed as milligrams of gallic acid equivalents per gram of bayberry leaf.
Determination of PDs. Determination of PDs was conducted according to the modified vanillin assay (Sun et al., 1998). Sample solution (1 mL) was added with 2.5 mL of 1% (w/v) vanillin in methanol and 2.5 mL of 20% (v/v) H2SO4 in methanol. The mixture was reacted at 30°C for 15 min. The absorbance of the mixture was measured at 500 nm by using a spectrophotometer, and content of PDs were expressed as milligrams per gram of (+)-catechin equivalent.
Determination of hot water extracts. The hot water extraction solution (5 mL) was added to weighing disks. The weighing disks were placed in an air dry oven at 50°C to remove water. Weight of extract was weighed and contents of hot water extract in bayberry leaves were calculated.
NPHPLC-DAD and NPHPLC-UV-ESIMS analysis PDs in hot water extraction were purified according to the reported methods with some modification (Gu et al., 2002; Hammerstone et al., 1999). Solution of water extraction was successively washed with cyclohexane (3 × 80 mL) and dichloromethane (3 × 80 mL). The organic solvents were evaporated under vacuum. The aqueous phase was lyophilized to dryness, dissolved in 30% methanol, and loaded on Sephadex LH-20 column (10 × 100 mm). The column was washed with 30% methanol (100 mL) and 70% acetone. Eluant of 70% acetone (50 mL) was recovered for analysis of PDs.
A modification of NPHPLC-DAD and NPHPLC-UV-ESIMS methods were used for PDs analysis (Hellström and Mattila, 2008; Kelm et al., 2006). NPHPLC-DAD was performed on a Waters platform, composed of a Waters 2695 HPLC and a Waters 2998 photodiode array detector. UV-Vis absorption spectra were recorded online during HPLC analysis. Spectral measurements were made at 200 – 400 nm. NPHPLC-UV-ESIMS analysis was done by Agilent 1100 Series and electrospray ionization in the negative mode on ESQUIRE3000 PIUS (BRUKER). Samples were separated on a 250 × 4.6 mm i.d., 5 µm, Silica Luna column (Phenomenex Inc., Darmstadt, Germany) and detected at 280 nm. Mobile phase consisted of dichlormethane (A), methanol (B), and acetic acid and water (1:1, v/v) (C). The linear gradient was as follows: 14 ∼ 23.6% B (0∼20 min), 23.6 ∼ 40% B (20 ∼ 50 min), 40 ∼ 86% B (50 ∼ 55 min), 86% B (55 ∼ 60 min), 86 ∼ 14% B (60 ∼ 65 min). A constant 4% C was kept throughout the gradient. Sample of 10 µL was analysis at a flow rate of 1 mL/min at 37°C. An external standard of EGCG was used for quantification. Quantification was based on UV detection using flat baseline integration.
Calculation of mDP The mDP of hot water extracts was measured and calculated by our published method (Yang et al., 2011 b). Briefly, the lyophilized samples (5 mg) were reacted with 0.2 N HCl and 50 g/L phloroglucinol at 60°C for 1 h. The reaction products were analyzed and determined by HPLC-DAD immediately on a ZORBAX SB-C18 (Agilent, USA) column (250 × 4.6 mm, 5 µm) at 280 nm. The mDP of the samples was calculated according to the following formula on a molar basis:
 |
Evaluation of antioxidant activities of hot water extract DPPH assay. This assay was based on the method of Gorinstein et al. (2004). Briefly, 0.2 mL of sample solutions was added to 2.8 mL of 0.1 mmol/L DPPH radical solution, which was freshly made. After 30 min of incubation at 27°C without light, the absorbance at 517 nm was measured. The control was prepared as above with water, and methanol was used for baseline correction. Radical-scavenging activity was expressed as percentage inhibition and was calculated using the formula
 |
Ferric reducing antioxidant power test. The ferric reducing ability of water extract was measured according to a modified protocol developed by Benzie and Strain (1996). To prepare the FRAP reagent, a mixture of 0.1 M acetate buffer (pH 3.6), 10 mM TPTZ, and 20 mM ferric chloride (10:1:1, v/v/v) was made. The FRAP reagent (4.9 mL) was added to 0.1 mL of the sample solutions. Readings were recorded on the Shimadzu UV-2550 spectrophotometer at 593 nm, and the reaction was monitored for 10 min. Then the mean absorbance values were plotted against the concentration of sample solutions.
Statistic analysis All samples were prepared and analyzed in triplicate. To verify the statistical significance of parameters, the values of means ± standard deviation (SD) were calculated. To compare several groups, analysis of variance (ANOVA) was used. The p value were used to show the significance [SPSS for Windows, Release 16.0.0 (Sep. 2007, SPSS Inc.)]. Probability values of p < 0.01 and p < 0.05 were adopted as the criterion for significant differences.
Distribution of polyphenols and PDs in different bayberry leaves Bayberry leaf of Biqi and Dongkui gave the same trend but different results in terms of the relative amount of polyphenols and PDs (Table 1). In a general way, immature leaves contained significantly more polyphenols and PDs (194.04 mg/g and 117.54 mg/g for Biqi, 196.26 mg/g and 100.95 mg/g for Dongkui) than intermediate and mature leaves of Biqi and Dongkui. Contents of polyphenols and PDs in intermediate leaves were lowest. Difference in polyphenols and PDs contents between two cultivars were relatively little. Therefore, immature leaves of two cultivars were selected as materials for water extraction.
| NO. | Bayberry leaf | Content of total phenolics | Content of prodelphinidins | |
|---|---|---|---|---|
| 1 | Biqi | Immature | 194.04 ± 4.11a | 117.54 ± 1.96a |
| 2 | Intermediate | 81.38 ± 0.43d | 38.40 ± 0.80e | |
| 3 | Mature | 117.15 ± 0.45c | 64.22 ± 1.86d | |
| 4 | Dongkui | Immature | 196.26 ± 3.26a | 100.95 ± 6.32b |
| 5 | Intermediate | 115.67 ± 4.10c | 63.67 ± 1.52d | |
| 6 | Mature | 131.69 ± 1.91b | 77.24 ± 1.58c | |
Significant difference (p < 0.05) was indicated by different letters in a same column.
Effect of hot water extraction on contents of total phenolics, PDs, and extracts Contents of total phenolics, PDs, and extracts under different extraction conditions in Fig. 1 showed similar change trend. Generally speaking, contents of total phenolics, PDs, and extracts of Biqi and Dongkui increased with temperature raise from 25 to 100°C and time extension from 10 to 30 min. Extraction temperature showed significant effect but not extraction time. When extracted at higher temperature for longer time, the contents of total phenolics, PDs, and extracts might decreased slightly. The highest contents of total phenolics (∼250 mg/g for Biqi, ∼270 mg/g for Dongkui) and extracts (∼140 mg/g for Biqi, ∼160 mg/g for Dongkui) of Biqi and Dongkui were similar. But the two cultivars were different in terms of PDs contents (∼70 mg/g for Biqi, and 120 mg/g for Dongkui), indicating that they were different sources of PDs.

Contents of hot water extract (1), total phenolics (2), and prodelphinidins (3) of bayberry leaves (mg/g, DW) (A: Biqi; B: Dongkui). Bars indicate the standard deviations.
Qualitative analysis of PDs by NPHPLC-UV-ESIMS Because of the lack of PDs standards, identification of PDs by NPHPLC-UV-ESIMS before quantitive analysis of PDs was very necessary. NPHPLC chromatograms of bayberry leaf PDs are shown in Fig. 2. Fig. 2B is detailed chromatograms of that in the circle in Fig. 2A. PDs were eluted from the column after 30 min. Dimers (DP2) of PDs can be nicely separated by NPHPLC while trimers (DP3) and tetramers (DP4) cannot distinctly separated, indicating higher complexity in their structures. Other polymers with higher DP than four cannot separated totally and appeared as a single peak (PP) at the end of elution.

Normal phase HPLC chromatogram of bayberry leaf prodelphinidins detected at 274 nm (A: full spectrum; B: part spectrum in circle). PP, polymeric prodelphinidins with DP > 4; DP, degree of polymerization; Numbers of 762, 914, etc. were molecular weight of individual prodelphinidins
Characterization of the separated individual PDs is shown in Table 2. All detected individual PDs were B-type PAs. Two single dimers were detected unambiguously. They were PDs dimers consisting of EGC+EGCG and 2EGCG, respectively. The detected trimers can be classified into three groups according to their molecular weight (Table 2). They were PDs trimers consisting of EGCG+2EGC, 2EGCG+EGC, and 3EGCG. Three groups of tetramers were also isolated (Table 2). They were PDs tetramers containing units of 2EGCG+2EGC, 3EGCG+EGC, and 4EGCG. There were more than one compounds in each group of trimers or tetramers. These compounds in the same group had same molecular weight and different space structures.
| Degree of polymer | Collecting time period (min) | λmax (nm) | Molecular weight | Prominent ions (m/z) | Tentative identification |
|---|---|---|---|---|---|
| 2 | 30∼35 | 272.3 | 762 | 1523.8, 761.3, 591.2, 423.1 | EGC+EGCG |
| 273.5 | 914 | 1827.8, 913.5, 743.3, 573.2 | 2EGCG | ||
| 3 | 35∼44 | 272.3 | 1066 | 2132.9, 1065.6, 913.4, 761.3, 423.4 | EGCG+2EGC |
| 272.3 | 1218 | 1217.5, 1065.4, 913.4, 761.2, 594.9 | 2EGCG+EGC | ||
| 273.5 | 1370 | 1369.6, 1217.5, 1065.3 | 3EGCG | ||
| 4 | 44∼50 | 272.3 | 1522 | 1521.7, 1369.4, 1217.5 | 2EGCG+2EGC |
| 272.3 | 1674 | 1673.7, 1521.6, 1369.5 | 3EGCG+EGC | ||
| 273.5 | 1826 | 1826.8, 1673.7, 1521.4, 1369.3, 1217.2 | 4EGCG |
Effect of Hot Water Extraction on Composition of PDs Each content of PDs (Fig. 3) determined by NPHPLC-DAD was lower than that determined by vanillin assay (Fig. 1) even though the PDs were extracted from same materials under same extraction condition. But both results obtained by the two methods showed similar trend. In main cases, contents of PDs extracted at high temperature were higher than that extracted at low temperature in both cultivars. PDs contents in Biqi increased (from ∼0.8 mg/g to ∼2 mg/g) at 25°C but decreased at higher temperature than 50°C with the prolonged time from 20 to 30 min. However, contents of PDs in Dongkui were increased at any temperatures with time ranging from 10 to 30 min. PDs contents of Dongkui were more than two times as much as that of Biqi under same extraction condition, indicating composition of PDs in the two cultivars were different.

Contents of prodelphinidins in bayberry leaves extracted by hot water and determined by NP-HPLC (mg/g, DW) (A: Biqi; B: Dongkui)
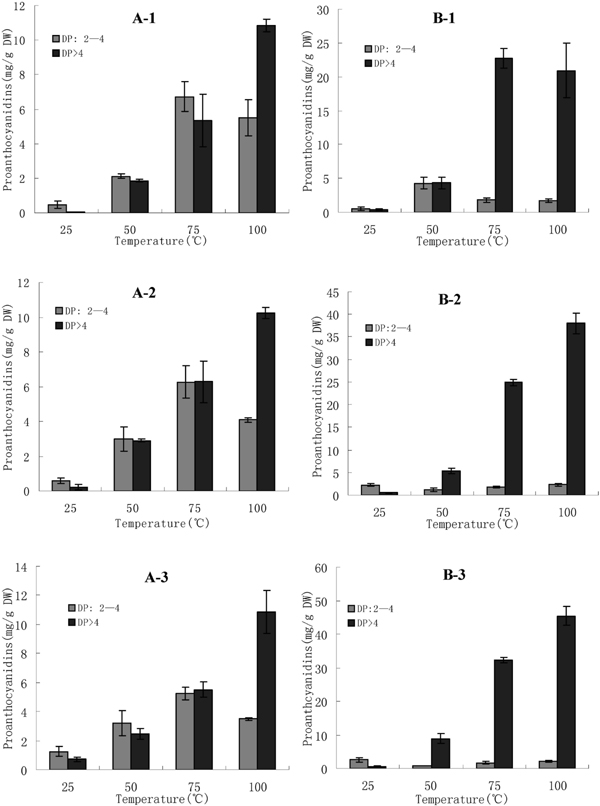
Fig. 4 shows effect of hot water extraction on contents of PDs with different DP. Letter A and B represent Biqi and Dongkui cultivars, respectively. Numbers (2, 3, 4, 5) following the letters represent PDs dimers, trimers, tetramers, and polymers with DP > 4, respectively. Results of the two cultivars were different. Contents of dimers (∼4 mg/g) and trimers (∼2.2 mg/g) of Biqi was highest at 75°C while contents of tetramers (∼1.7 mg/g) and other polymers (∼11 mg/g) was highest at 100°C. The highest content of dimers (∼4 mg/g) in Dongkui existed at 50°C. Other polymers showed the highest content nearly at 100°C. Contents of polymers with DP < 4 in Biqi were decreased with prolonged time from 10 to 30 min. It was interesting that this trend could only be found for dimers of Dongkui. This phenomenon might be caused by different composition and space structure of trimers and tetramers in different materials.

Contents of prodelphinidins with different degree of polymerization (DP) in bayberry leaves extracted by hot water. (A: Biqi; B: Dongkui; 2: DP = 2; 3: DP = 3; 4: DP = 4; 5: DP > 4.)
Effect of hot water extraction on composition of PDs is shown in Fig. 5. Letter A and B in the figure also represent Biqi and Dongkui cultivars, respectively. But the current numbers of 1, 2, and 3 following the letters stand for extraction time of 10 min, 20 min, and 30 min, respectively. PDs can be divided into oligomers (DP ≤ 4) and high polymers (DP > 4). Extraction temperature affected more than extraction time set on oligomers and high polymers. In a certain temperature range (25 ∼ 75°C), contents of oligomers increased with the temperature rise. On the contrary, when extracted at high temperature (75 ∼ 100°C) they decreased as the temperature increased. In terms of high polymer, the higher the extraction temperature, the higher the content. The oligomer contents in Biqi were considerable, just like the high polymers in Dongkui. Therefore, the mDP of Dongkui (nearly 8 ∼ 9) was larger compared with that of Biqi (nearly 2.6 ∼ 4) under the same extraction condition (Fig. 6).
Effect of temperature and time on composition of bayberry leaf prodelphinidins (A: Biqi; B: Dongkui; 1: 10 min; 2, 20 min; 3: 30 min)

The mDP of prodelphinidins in bayberry leaves extracted by hot water (A: Biqi; B: Dongkui; mDP: average degree of polymerization)
Antioxidant Activity of Hot Water Extracts DPPH (Fig. 7A) and FRAP (Fig. 7B) assays were employed to evaluate the antioxidant activity of hot water extracts. DPPH results showed a trend that the higher temperature, the lower EC50 value, indicating that ability of scavenging DPPH radical of the extract enhanced with increase of temperature. The lowest EC50 of Biqi and Dongkui was ∼0.013 mg and ∼0.009 mg, respectively. In FRAP assay, values showed the opposite trend, suggesting that reducing power of the extracts increased with the increase of temperature. Both results of the two assays proved that hot water extract possessed antioxidant activity and the ability increased with temperature raging from 25 to 100°C. Extraction time change also affected little on antioxidant activity.

DPPH scavenging activity (1) and ferric reducing ability (2) of hot water extract of bayberry leaves (A: Biqi; B: Dongkui)
Polyphenols are plant secondary metabolites which protects plant from external injury (Maldonado et al., 2015). Therefore, most of the polyphenols distribute on plant epidermis, immature leaf, bud, and seed. The results above, immature leaves of both cultivars had higher contents of total polyphenols and PDs than that of intermediate and mature leaves, also proved the point. Secondly, PAs in different plants are various. For example, PAs in grape seed and cranberry are procyanidin type while barley PAs are procyanidin type and PDs type (Foo et al., 2000; McMurrough and Baert, 1994; Prieur et al., 1994). Although PAs in Biqi and Dongkui are PDs type (Yang et al., 2011 b), their composition of PDs are different. Biqi contained more oligomers and less polymers than Dongkui. Thus the proper materials should be selected in practical application. Besides, content of PDs were affected by determination methods used. Results of our present study showed contents evaluated by using vanillin assay were apparently higher than that determined by NPHPLC method. In vanillin assay, other compounds with similar molecular groups would interfere (Hömmer and Schreier, 2008). When being analyzed using NPHPLC method, PDs were purified, separated from other compounds, and determined. Therefore, the result was more close to the actual value.
NPHPLC-UV-ESI/MS is a powerful tool for qualitative and quantitative analysis of PAs. PAs are a mixture with complicated structure and composition. Isolation and purification of PAs monomer were very difficult especially when PAs had a high DP. Therefore, PAs standards were very lacking. At present, only procyanidin dimers and trimers could be purchased. Therefore, NPHPLC-ESIMS was usually used for identification and analysis of PAs. Moreover, identification of PAs was essential before determination of each single PAs compounds further. In NPHPLC, PAs are eluted in the order of increasing molecular mass (Pei et al., 2015). Mobile phases and elution gradient were key factors affecting separation. Dichloromethane, methanol, hexane, acetone, and ethyl acetate were commonly used mobile phases (Fu et al., 2014; Pei et al., 2015). Little organic acids such as formic acid and acetic acid were also added to improve the resolution (Hömmer and Schreier, 2008; Sarnoski et al., 2012). It was reported that PAs with a DP more than 10 could be separated with a good resolution (Hellström and Mattila, 2008). In present research, dichloromethane, methanol, acetic acid, and water were used as the mobile phase. To separate more PDs as far as possible, the gradient was adjusted. However, only dimers, trimers and tetramers could be separated. Other polymers with higher DP than four cannot be separated totally and appeared as a single peak at the end of elution. The phenomenon might attribute to more complex structure and higher molecular weight of bayberry leaf PDs than other procyanidins. The eluting molecules of NPHPLC device were then analyzed by ESI/MS. Special ions generated such as retro-Diels-Alder fission ions and pseudomolecular ions were used for identification of PDs (Friedrich et al., 2000; Hellström et al., 2007). Because PDs were purified before NPHPLC-UV-ESI/MS analysis and structure composition of PDs were known already, the method could be used for quantification of PDs.
In China, hot water was traditionally used for cooking tea and Chinese medicine. Functional components such as polyphenols and polysaccharides were extracted from the materials by hot water (Pasrija and Anandharamakrishnan, 2015). Different materials, different extraction conditions. For example, extraction time of tea was commonly shorter than that of Chinese medicine (Sharpe et al., 2016; Yang and Zheng, 2012). Bayberry leaf, twig, and bark were used as traditional folk medicine for a long time (Matsuda et al., 2002). PDs were one of functional compounds in bayberry leaves. But the composition of PDs in infusions under different extraction condition was not clear and which extraction condition should be chosen was ambiguous. The present results showed that Biqi contained more PDs oligomer than Dongkui. In terms of PDs polymer, the results were opposite. More PDs oligomers could be extracted from bayberry leaf at appropriate low temperature (∼ 75°C) for short time (∼10 min). Higher extraction temperature and longer time, more PDs polymers. Therefore, extraction condition you chosen to extract PDs from bayberry leaf should conform to your purpose.
It was well known now that antioxidant activity of compounds in vivo was greatly affected by gastrointestinal tract digestion (Rufian-Henares and Delgado-Andrade, 2009). Research methods in vitro were thought to be inaccurate to reflect the antioxidant activity of compounds in vivo (Lopez-Alarcon and Denicola, 2013). However, the in vitro methods were very easy and convenient. A more important fact was that these methods could calculate the antioxidant activity of compounds when they used as antioxidant in foods. In order to obtain reliable results, more than one assays in vitro such as DPPH, FRAP, ORAC and ABTS assays were usually used for evaluating the antioxidant capacity (Alam et al., 2013; Zou et al., 2016). In present study, DPPH and FRAP methods were employed to determine antioxidant activity of hot water extract of bayberry leaf. Results indicated that the extract had antioxidant activity. Our previous results revealed a high, positive and linear correlation between bayberry leaf antioxidant activity and total phenolics or PDs. Furthermore, PDs were proved to be the major factor accounting for the antioxidant activity of bayberry leaf (Yang et al., 2014). The antioxidant activity and mechanism of bayberry leaf PDs would be studied systematically further.
In summary, bayberry leaf PDs in hot water extract were identified by using NPHPLC-UV-ESI/MS and effect of hot water extraction on composition and antioxidant activity of PDs were studied. Oligomers of PDs with DP < 4 could be separated and identified by NPHPLC-UV-ESI/MS. Temperature and material significantly affected the contents and composition of PDs in hot water extracts. Therefore, appropriate materials and extraction condition were suggested for your aims.
Acknowledgments This project was supported by National Natural Science Foundation of China (K3050215202) and a special fund for basic scientific research of Northwest A&F University (Z109021506).