2017 Volume 23 Issue 1 Pages 119-127
2017 Volume 23 Issue 1 Pages 119-127
There is an increasing interest in developing renewable and degradable gelatin films for various applications. However, gelatin has to be modified before use due to its poor mechanical properties, sensitivity to water and microorganisms. In this work, gelatin films were modified physically and chemically. Effects of the molecular size of the modifying agents on the properties of the structure and properties of gelatin films were discussed. It was found that alcohols with higher molecular weight endowed the gelatin films with reduced migration ratio in water, showing an excellent plasticizing stability. Films chemically modified with macromolecule dialdehyde starch (DAS) showed obviously lower swelling ratio than that modified with small molecule glutaraldehyde (GTA). The present work indicated that the plasticizers and crosslinking agents with proper molecular size and molecular weight endowed the physically and chemically modified gelatin films with superior physical properties, providing useful information for the modification of gelatin and other biopolymers.
Gelatin is an irreversibly hydrolyzed form of collagen, which is the main structural protein of the various connective tissues in animals (Schrieber and Gareis, 2007; Gomez-Guillen et al., 2011). Gelatin can be easily extracted from various sources, including the skin and bone from mammals as well as scales from fish (Zhou et al., 2006; Boran and Regenstein 2009; Nhari et al., 2012; Jakhar et al., 2014; Norziah et al., 2014; Le et al., 2015). As a biodegradable, biocompatible, low toxic material with various attractive properties, gelatin has been a modern product with a very wide range of uses, including food (Karim et al., 2008), pharmaceuticals (Djagny et al., 2001; Young et al., 2005), photography (Schrieber and Gareis 2007), and cosmetic manufacturing (Cheng et al., 2009). However, gelatin shows some obvious drawbacks such as water sensitivity, instability at about 37°C in liquid media and rather poor mechanical properties. To improve the above properties, it is important to modify gelatin through physical or chemical methods like lubricating and crosslinking (Jongjareonrak et al., 2006; Al-Hassan and Norziah, 2012; Amadori et al., 2015).
Plasticizers such as water and polyols (e.g. glycerol, sorbitol, etc.) were usually used to improve the brittleness of gelatin and gelatin-based composite films (Jongjareonrak et al., 2006; Vieira et al., 2011; Al-Hassan and Norziah 2012), which can lubricate the gelatin molecular chains and improve the flexibility of gelatin products. Glycerol is probably the most widely used plasticizer due to the low cost and good safety. However, glycerol is a kind of small molecule and easily migrates towards surface of the product. Moreover, glycerol can be dissolved in water at any proportion. The excessive hydrophilic property of glycerol results in an unstable plasticizing effect especially in moisture environment (Thomazine et al., 2006).
Gelatin and gelatin-based materials can be crosslinked using chemical (e.g. chemically crosslinking), physical (e.g. lubrication) or biological (e.g. enzyme crosslinking) methods to further improve its stability and mechanical properties (Chang et al., 2012; Huang et al., 2012; Mu et al., 2012; Otoni et al., 2012; Falconi et al., 2013; Teddei et al., 2013; Amadori et al., 2015). Depending on the degree of crosslinking, gelatin with improved properties can be obtained, with increased mechanical strength, decreased swelling ability, and improved resistance to microorganisms. Glutaraldehyde (GTA) is by far one of the most widely applied chemical crosslinking agents, especially for proteins, due to its low cost and excellent efficiency on the stabilization of collagenous materials, which enables achieving strength and water resistance of the obtained structure (Bigi et al., 2001; Alves et al., 2011). However, GTA has tendency of self polymerization, which easily reduces the crosslinking efficiency and usually displays a yellow color of the products. GTA is also known to elicit cytotoxicity, and calcification of GTA treated protein, e.g. collagen, on implantation has caused great concern (van Luyn et al., 1995). Moreover, GTA with high saturated vapor pressure is easy to volatilize a large amount of irritating substance, which is apparently harmful for the environment and the health of the operators. Dialdehyde starch (DAS) is a polymeric aldehyde obtained by the reaction of native starch with periodic acid, which has shown low toxicity to rats by oral, dermal, and respiratory routes of introduction (Wilson, 1959).
Gelatin based films may find applications in the food packaging sector. The highly water sensitivity and poor mechanical behavior usually limit its application. Investigation on the physical and chemical modification of gelatin films is important. In the present work, comparative work has been carried out to investigate the physical and chemical modification for gelatin films, aiming at the investigation of the influence of the molecular size or molecular weight on the plasticizing stability and crosslinking effect for gelatin films, expecting provides useful information for the modification of gelatin and other biopolymers such as collagen.
Materials Gelatin granules (biochemical reagent) were supplied by Tianjin Chemicals Co., Tianjin, China. Dialdehyde starch (medical grade) was purchased from Tai'an Jinshan Modified Starch Co., Shandong, China. Other chemical agents were all analytically pure without further purification. Poly(ethylene glycol) (PEG) with different molecular weight, e.g. PEG200, PEG400 and PEG800, used as received without pretreatment.
Physical Modification of Gelatin Films 30 g gelatin was soaked in 120 mL distilled water at 40°C for 45 min, and then heated to 60°C until the solution became homogeneous. Plasticizers, e.g. glycerol, PEG200, PEG400 and PEG800, were added at various contents (shown in each figure). The solution was stirred for 2 h and then 120 mL solution were poured into a self-made mold and dried in air to get the physical modified gelatin film. The samples were conditioned at 25°C and relative humidity (RH) about 65% (controlled by saturated NH4NO3 solution) for at least one week to constant weight. The average thickness of the film is about 0.07 mm. The water content of the films is about 20 wt%.
Chemical Modification of Gelatin Films The 20 wt% gelatin solution with PEG400 (content of 20 wt% based on dry gelatin) as a plasticizing agent was prepared by the above method. Dialdehyde starch (DAS, 7.4 wt% based on dry gelatin) or glutaraldehyde (GTA, 1.5 wt% based on dry gelatin) as crosslinking agent were added into solution and reacted at 60°C and pH 9. Gelatin modified by DAS and GTA was denoted as DAS-gelatin and GTA-gelatin, respectively. The solution was poured into a self-made mold and dried in air to get the crosslinked gelatin films.
FTIR characterization The infrared spectra of the samples were recorded by using a FTIR Nicolet 6700 (Thermo Scientific). 32 cans were signal-averaged with a resolution of 4 cm−1.
Mechanical tests Mechanical testes of the dumbbell samples were measured with a universal tensile tester, CMT5140 (Shenzhen Sansi Co., China). The tests were carried out at a crosshead speed of 50 mm/min at room temperature and humidity. The average of at least 5 tests was reported.
Plasticizer migration behaviors Samples were conditioned at 25°C and RH about 65% (controlled by saturated NH4NO3 solution) for one week to a constant weight. The sample was cut into size of 1 cm × 1 cm, which has been weighed accurately (denoted as m0). After being immersed in ice water for at different time period time, the sample was taken out at time intervals and dried in oven for at least 24 h till constant weight (denoted as m1). The plasticizer migration percentage R was calculated as below.
 |
Swelling behaviors The dynamic water absorption of the samples was measured by a gravimetric procedure. The samples were conditioned at 25°C in a desiccators containing P2O5 for one week. A sample, which has been weighed accurately, was immersed in ice water. The sample was taken out at different time intervals and the surface was dried quickly with filter paper and the weight gain was measured. Water absorption was calculated as weight in the equilibrium per weight of dry polymer and expressed as the swelling ratio.
 |
where Q is the swelling ratio, m0 and mt denote the original weight and weight at time t, respectively.
SEM The samples were immersed and broken in liquid nitrogen, coated with a thin layer of gold and examined by scanning electron microscopy (SEM, Phenom Prox, The Netherlands).
Physical modification of gelatin films
Migration of plasticizers The migration percentage of various plasticizers in physically modified gelatin films measured in ice water is shown in Fig. 1. Due to the high molecular weight of gelatin, the glycerol and PEG can be regarded as small molecules. It can be assumed that only the plasticizers (glycerol and PEG) migrate out of the gelatin films in the ice water during the testing time (< 60 min).

Migration percentage of plasticizers in gelatin films in ice water (a) glycerol, (b) PEG 200, (c) PEG 400, (d) PEG 800
It is seen that, generally speaking, the migration percentage of plasticizers in the gelatin films increases with the soaking time. This may be related to the change in the intermolecular interaction in the system. The main interaction between the plasticizers and gelatin is hydrogen bonds. With the soaking time increasing, water tends to penetrate into gelatin film. The plasticizers tend to form hydrogen bonding preferentially with water molecules, therefore, part of the hydrogen bonds between plasticizers and gelatin are broken. As a result, plasticizer molecules migrate out of the gelatin film and dissolve in water. It is seen from Fig. 1a that the migration percentage of glycerol is quite high, especially at high glycerol content (> 30 wt%). It indicates that the hydrogen bonding formed between gelatin and glycerol tends to be saturated at glycerol content of 30 wt%. At higher content, the redundant glycerol exists in gelatin in dissociated form and dissolves quickly in water, and therefore, the samples show high glycerol migration percentages. Compared with glycerol, PEG applied in this work (PEG 200, PEG 400, PEG 800) has much lower migration ratio and exhibits stable plasticizing properties. This is may be the result that PEG has higher molecular weight and less molecular mobility compared with glycerol. It is seen from Figs. 1c and 1d that at higher plasticizer content (> 30 wt%), the plasticizers migration percentage of PEG 400 and PEG 800 decreases. This may be that with the molecular weight of PEG increases, the polarity of PEG decreases, and the dissolving ability of PEG in water decreases. Moreover, with the molecular weight increases, the PEG chains tend to entangle with gelatin macromolecules. The entanglement hinders the mobility of PEG chains and therefore reduces the PEG migration percentage. These results indicate that the molecular size of plasticizers affect the plasticizing stability.
Mechanical properties of physically modified gelatin films Fig. 2 presents the mechanical properties of gelatin films with different plasticizers. It is seen that with the increase of plasticizer content, the tensile strength of the modified gelatin films decrease and the elongation at break increase. It is accordant with the common rule of lubricated polymer materials. At the same plasticizer content, the tensile strength of PEG modified gelatin films is always higher than the corresponding glycerol modified film. Furthermore, PEG 400/gelatin shows higher tensile strength than PEG 200/gelatin films. It indicates that plasticizer with higher molecular weight results in better mechanical properties. However, with PEG molecular weight increasing to 800, a decrease in tensile strength of PEG/gelatin film is observed compared with PEG 400. It is seen that the plasticizer molecular weight does not increase the tensile strength all along. It may be that, at higher PEG molecular weight, PEG chains tend to entangle with each other and therefore result in a slight phase separation in PEG lubricated gelatin system. Due to the fact that PEG 800 has lower mechanical strength than matrix, the redundant plasticizer decreases the tensile strength (Laohakunjit and Noomhorm 2004).

Mechanical properties of plasticized gelatin films.
The elongation at break results shown in Fig. 2b indicates that the incorporation of plasticizers increases the flexibility of gelatin films steadily. At low content (<10.0 wt%) glycerol is more effective to increase the elongation at break. However, at higher content, the increase in elongation at break of PEG lubricated system increases more effectively.
Chemical modification of gelatin films
FTIR of chemically modified gelatin films Fig. 3 shows the FTIR spectra of gelatin and GTA crosslinked gelatin. It is generally accepted that GTA can be explained through the reaction of the aldehyde functional groups with free nonprotonated ε-amino groups of lysine or hydroxylysine through a nucleophilic addition-type reaction. More specifically, the first step of the reaction involves the nucleophilic addition of the ε-NH2 groups to the carbonyl groups (C=O) of the aldehyde to form a tetrahedral unstable intermediate called carbinolamine. In a second step, protonation of the –OH group followed by loss of a water molecule yields the conjugated Schiff bases (Farris et al., 2010). In the spectrum of pure gelatin, we can observe the typical bands such as O-H stretching at 3408 cm−1 for the amide A, 1653 cm−1 for the amide I mainly associated with the C=O stretching vibration, N-H bending vibration at 1543 cm−1 for the amide II and N-H deformation at 1238 cm−1 for the amide III band (Azami et al., 2002). When gelatin is crosslinked by GTA, a Schiff base is formed. C=N stretching vibration of Schiff based is in the 1650 – 1600 cm−1 region, however, this peak is covered by the amide I band of gelatin. In GTA-gelatin spectrum, strong peaks at 1103 cm−1 corresponding to C-O stretching vibration and 948 cm−1 corresponding to hydrogen-pout-of-plane vibration of the N-H group are found in GTA crosslinked gelatin spectrum (Farris et al., 2010), which indicates that GTA has successfully crosslinked gelatin.

FTIR spectra of pure gelatin and GTA-gelatin.
Fig. 4 shows the FTIR spectra of gelatin, DAS and DAS crosslinked gelatin. Similarly, a strong peak at around 1106 cm−1 corresponding to C-O stretching vibration and 947 cm−1 corresponding to hydrogen-pout-of-plane vibration of the N-H group are also found in the spectrum of DAS crosslinked gelatin, indicating a successful crosslinking reaction between DAS and gelatin.

FTIR spectra of pure gelatin, pure DAS and DAS-gelatin.
Swelling behavior of chemically modified gelatin films Due to the large amount of hydrophilic groups on gelatin macromolecular chains, gelatin tends to highly swell in water. In food packaging application, gelatin films are not expected to be highly swollon when contact with water rich material. The improvement in water resistance of gelatin films is important. Fig. 5 shows the swelling curves of GTA and DAS crosslinked gelatin films in ice water. Since the uncrosslinked gelatin films break into pieces in ice water during the swelling experiments, the swelling curve of pure gelatin is not shown in Fig. 5.
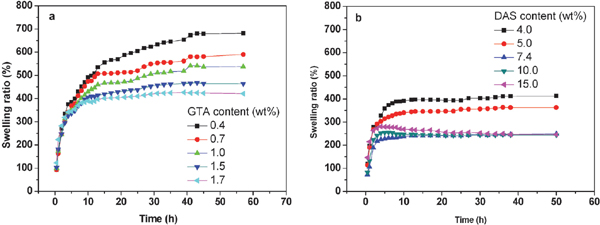
Swelling behavior of gelatin films crosslinked by GTA (a) and DAS (b).
From Fig. 5a it is seen that all adsorption curves of GTA-gelatin films are characterized by three zones: fast adsorption zone at short time (t < 5 h), a rapid increase in water uptake occurs; sequential slow adsorption zone at extended time (5 h < t < 15 h), the water uptake increases gradually; and the plateau at long time (t > 15 h), the adsorption rate hardly changes, corresponding to an adsorption equilibrium. It is seen from Fig. 5a that with the GTA content increasing, the water uptake at equilibrium decreases gradually. It indicates that the crosslinking by GTA endows the gelatin with three-dimensional network structure and the penetration of water is therefore hindered. It is seen from Fig. 5a that the swelling ratio of GTA-gelatin decreases with the increasing of GTA. It indicates that at low GTA content, the crosslinking reaction forms a GTA-gelatin network with a loose structure. With the increasing of crosslinking agent content, the reaction points between GTA and gelatin increases, resulting a dense network. With the water molecules enter into the network of GTA-gelatin, there are opposing forces between the network of GTA-gelatin and water. The dense network has a smaller swelling ability and a bigger repelling force to water. Therefore, the GTA-gelatin with higher GTA content has smaller swelling ratio.
The swelling curves of DAS-gelatin films are illustrated in Fig. 5b. It is seen that at low DAS content of 4.0 wt%, 5.0 wt% and 7.4 wt%, the swelling ratio of DAS-gelatin films increases rapidly in the first 5 h and reaches at equilibrium much faster that GTA-gelatin, indicating that DAS-gelatin samples swell less in water. Since DAS is a macromolecular multiple aldehyde, one DAS macromolecular chain can react with several gelat in macromolecular chains and forms a denser crosslinking network (as illustrated in Fig. 6) and therefore prevents the water penetration more effectively. Also, due to the crosslinking in the gelatin structure, hydrophilic sites along gelatin chains become less exposed and less accesible to water molecules (Martucci and Ruseckaite, 2009; Martucci and Ruseckaite, 2010). However, it is seen from Fig. 5b that the swelling ratio at equilibrium no longer changes at DAS content higher than 7.4 wt%. It is deduced that a further increase of DAS content will not contribute to the crosslinking degree of gelatin.

Schematic drawing of macromolecular interaction between DAS and gelatin
Comparing Fig. 5a and 5b it is seen that the swelling ratio of DAS-gelatin films is much smaller than that of GTA-gelatin. It indicates that DAS can endow the gelatin with improved structural stability and therefore less sensitive to water. This is favorable for the applications in the environment with high humidity.
Mechanical properties of chemically modified gelatin films Fig. 7 shows the mechanical properties of GTA crosslinked gelatin films. It is seen that the crosslinking by GTA and DAS drastically increases the tensile strength and elongation at break of gelatin films. For GTA crosslinked gelatin, at content of GTA below 1.5 wt%, the tensile strength of the films increases with the increasing of GTA content, while the elongation at break decreases. However, at GTA content higher than 1.7 wt%, the tensile strength tends to decrease as shown in Fig. 7a. This is attributed to the local excessive crosslinking in the gelatin. At high GTA content, the local excessive crosslinking points in the gelatin act as defects or stress concentration points and impair the mechanical properties. Fig. 7b shows that the tensile strength increases with the addition of DAS. At DAS content of 7.4 wt%, the tensile strength of DAS-gelatin increases 36% compared with pure gelatin, suggesting the effectively cross-linking between gelatin and DAS. However, the tensile strength of DAS-gelatin films decreases at DAS content higher than 7.4 wt%. This probably indicates that the crosslinking reaction between gelatin and DAS has reached a “saturation” point at high DAS content. Although additional reactive groups might have been available along gelatin macromolecular chains, steric hindrance most likely prevented aldehydic functions on the polymeric DAS from interacting with remaining reactive amino acid groups. Due to the polymeric nature of DAS, macromolecular chains aggregation tends to take place in DAS-gelatin system and the starch rich domains exerts a negative effect on the mechanical properties of the film as local stress concentration points. Figs. 7c and 7d show that the elongation at break increases at the beginning with the crosslinking while decreases at higher crosslinking agent contents. Crosslinking with low concentration of crosslinking agent (0.4 wt% GTA and 4.0 wt% DAS) increases the elongation at break drastically. It is seen that effective crosslinking endows the gelatin films with improved deformability. However, with higher crosslinking agent concentration, the elongation at break decreases gradually. It indicates that the excessive crosslinking at high crosslinking agent content decreases the deformability of gelatin films and therefore reduces the elongation at break.

Mechanical properties of chemically modified gelatin films
Compared comprehensively, the current work indicates that the plasticizers and crosslinking agents with proper molecular size and molecular weight endow the gelatin films with modified physical properties. The plasticizer migration ratio and mechanical results indicate that plasticizer with higher molecular weight owns preferable plasticizing stability. However, the molecular weight should be in an optimized range. Poly(ethylene glycol) (PEG) with proper molecular weight can endow the gelatin films with superior lubrication effect and plasticizing stability. Aldehyde can introduce effective crosslinking reaction between gelatin macromolecular chains and endow the gelatin films with drastically decreased swelling ratio in water and improved mechanical properties. The swelling ratio of DAS crosslinked gelatin films reaches at equilibrium much faster than GTA crosslinked ones. Gelatin films crosslinked with dialdehyde starch (DAS) has lower swelling ratio than glutaraldehyde (GTA) crosslinked ones, indicating that the macromolecular DAS is more efficient to improve the stability of gelatin in water. This investigation may provide useful information of physical or chemical modification of gelatin films in food packaging application.
Acknowledgement The financial support of this work by the National Natural Science Foundation of China (grant No. 51473150) and Henan Education Department of China (No. 13A430705 and 17HASTIT009) is gratefully acknowledged.