2017 Volume 23 Issue 1 Pages 91-100
2017 Volume 23 Issue 1 Pages 91-100
Methods for the production and isolation of the tea components teadenols A and B were developed because of their potential anti-metabolic syndrome activity. Aspergillus sp. (FARM AP-21280) was inoculated to a modified Czapek-Dox medium containing (-)-epigallocatechin 3-O-gallate (EGCG) and (-)-gallocatechin 3-O-gallate (GCG) and incubated at 25°C. The maximum contents of the teadenols were observed at 19 days, and the contents increased depending on the initial EGCG concentration. The addition of green tea powder to the medium enhanced the production of teadenols. In the modified Czapek-Dox medium containing 5.0% EGCG and 1.0% green tea powder, teadenols A and B were efficiently produced at 3.22 ± 0.23 and 4.49 ± 0.38 mg/mL, respectively. The teadenols were purified by sequential chromatography on Sephadex LH-20 and InertSustain™ C18 columns. After purification by preparative HPLC, 0.27 g teadenol A (29.7% recovery) and 0.45 g teadenol B (42.9% recovery) were obtained from the 970 mL culture filtrate.
Tea produced from the leaves of Camellia sinensis L. is one of the most popular beverages in the world, consumed not only for its health benefits, but also because of its pharmaceutical activity (Cabrera et al., 2006). The process used to manufacture tea from the harvested leaves determines its characteristics, including flavor, taste, and color (Wang et al., 2014). Traditionally, teas have been classified into three types—unfermented tea (green tea), semi-fermented tea (oolong tea), and fully fermented tea (black tea)—according to the degree of oxidation of the catechins by the polyphenol oxidase in the tea leaves (Wan et al., 2008). However, in addition to these three types of teas, many varieties fermented with microorganisms are produced in East Asian countries; such microbially fermented teas are called “post-fermented teas.”
Post-fermented teas are produced using a variety of specific microorganisms such as Aspergillus, Blastobotrys, Streptomyces, Rhizopus, Penicillium, and Saccharomyces (Zhao et al., 2015). Post-fermented teas have received much attention because of their health-promoting effects, such as reducing the risks of hyperlipidemia (Oyaizu et al., 2005), atherosclerosis (Hou et al., 2009b), and hypercholesterolemia (Yang and Koo, 1997). Moreover, post-fermented tea supplementation has been found to suppress fatty acid synthesis (Chiang et al., 2005) and NO production (Ku et al., 2010), and to inhibit infection by the influenza virus (Noguchi et al., 2008) and the proliferation of cancer (Zhao et al., 2011). The phenolic constituents in tea may contribute to these protective effects.
Teas are considered to be excellent natural sources of polyphenolic compounds, and compositional changes among the major tea polyphenols have been reported to depend on the processing procedures. The contents of catechins, as the major components in green tea, are higher than those in other types of teas because the polyphenol oxidase in the tea leaves and native microflora are inactivated by steaming or pan-frying (Toschi et al., 2000; Tanaka et al., 2011). In contrast, the catechins in fermented tea (black tea) are converted into theaflavins by oxidation with polyphenol oxidase in the tea leaves, thus reducing the catechin content (Friedman et al., 2009). Similarly, the catechins content in post-fermented teas markedly decreases during the fermentation process, although the catechins in the leaves of Camellia sinensis L. are decomposed by microbial enzymes and autoxidation (Jiang et al., 2011; Tanaka et al., 2011). The phenolic constituents of post-fermented teas differ (Jeng et al., 2007; Wu et al., 2007), and their contents are affected qualitatively and quantitatively by the microbial fermentation process. Interestingly, new compounds derived from the constituents in tea leaves appear during fermentation. Puerins A and B, two 8-C substituted flavan-3-ols, were isolated from Pu-erh tea (Zhou et al., 2005), as were four phenylpropanoid-substituted flavan-3-ols, puerins C-F (Tao et al., 2014). Two phenolic compounds, teasperol and teasperin, were isolated from Liu-pao tea, a traditional Chinese post-fermented tea (Kanegae et al., 2013). Furthermore, norisoprenoid and B-ring fission catechin (flavan-3-ol) derivatives, fuzhuanins A–F, were isolated from Chinese post-fermented teas (Luo et al., 2013; Luo et al., 2012; Zhu et al., 2015). Among these compounds, fuzhuanin B demonstrated significant anti-proliferative activity against HeLa cells (Luo et al., 2013). However, the biological activities of compounds derived from the constituents of tea by microbial fermentation have been poorly characterized.
Post-fermented teas are produced not only in China, but also in Japan, as exemplified by Yamabuki-Nadeshiko (YN), a post-fermented tea processed locally in Shizuoka prefecture in Japan using a specific microorganism, Aspergillus sp. (FARM AP-21280). Two newly isolated compounds from the tea, teadenols A and B (Wulandari et al., 2011b), are biosynthesized from (-)-epigallocatechin 3-O-gallate (EGCG) and (-)-gallocatechin 3-O-gallate (GCG), respectively (Wulandari et al., 2011c), and both compounds are likely produced from catechins by enzymatic reactions of Aspergillus sp. (FARM AP-21280). Teadenols A and B are detected specifically in post-fermented teas that have been fermented with Aspergillus sp.; therefore, both compounds have also been detected in Pu-erh tea, a traditional post-fermented tea. However, the contents of teadenols in Pu-erh tea are significantly lower than those in the Japanese post-fermented tea, YN (Ishimaru et al., 2012a; Ishimaru et al., 2012b; Wulandari et al., 2011a).
Generally, catechins in green tea exhibit antioxidant activity, and the contents of phenolic constituents reflect the tea's possible protective effects on the vascular endothelium. Although teadenol A does not exhibit any activity on vascular endothelial cells, it beneficially promotes the secretion of adiponectin in adipocytes and inhibits the secretion of protein-tyrosine phosphatase 1B. Considering these biological activities, teadenols are expected to have new anti-metabolic syndrome properties and may reduce the risk of obesity or diabetes (Yanagita et al., 2011a; Yanagita et al., 2011b). However, the teadenols A and B contents in traditional post-fermented teas are extremely low (Ishimaru et al., 2012a), and are insufficient to fully demonstrate the health-promoting effects described above.
Teadenols are potential new materials for the prevention of metabolic syndrome diseases (Yanagita et al., 2011a; Yanagita et al., 2011b) in a wide variety of applications, including teadenol-fortified teas and beverages. Therefore, it is necessary to develop sustainable methods for their large-scale preparation. The objective of this study was to optimize the Aspergillus sp. culture conditions for the enhanced production of teadenols, and establish chromatographic methods for their purification.
Materials Post-fermented tea (Yamabuki-Nadeshiko, YN) and concentrated extracts of the post-fermented tea were kindly provided by Riverson Co., Ltd. (Shizuoka, Japan). Two kinds of Pu-erh tea manufactured from tea leaves harvested in either China or Japan were purchased from Kenko-Seikatsu-Kenkyusho (Nara, Japan) and Arahataen Co., Ltd. (Shizuoka, Japan), respectively. Epigallocatechin gallate (EGCG, food-additive grade) was purchased from Tea Solutions, Hara Office Inc. (Tokyo, Japan). Green tea powder was purchased from Ochanomizukai Co., Ltd. (Shizuoka, Japan). Authentic EGCG and gallocatechin gallate (GCG) were purchased from Wako Pure Chemical Industries Ltd. (Osaka, Japan).
Preparation of authentic samples Authentic samples of teadenols A and B were prepared from the concentrated extracts of the post-fermented teas and the post-fermented tea (YN), respectively, according to the slightly modified method of Wulandari et al. (2011b). The structures of the purified teadenols A and B were confirmed by 13C and 1H nuclear magnetic resonance (NMR) spectroscopy.
Microorganism Aspergillus sp. (FARM AP-21280), provided by Riverson Co., Ltd., was used as the starter organism for the production of teadenols A and B. Aspergillus sp. (FARM AP-21280) was cultured on potato dextrose agar (PDA) medium slants at 25°C for 2 weeks. Portions of these slant cultures were inoculated to the liquid medium for tea culturing.
Culture conditions Modified Czapek-Dox medium composed of 0.3% NaNO3, 0.1% K2HPO4, 0.05% MgSO4·7H2O, 0.05% KCl, 0.001% FeSO4·7H2O, and 0.5% sucrose was used as the basal medium. After dissolving EGCG (0.1%, 1.0%, 2.0%, 5.0%, or 7.0%) in the above medium, the pH was adjusted to 6.0 with 4 N HCl. Tea powder (0.5%, 1.0%, 1.5%, or 2.0%) was suspended in the pH-adjusted medium. An aliquot of each medium (100 mL) was autoclaved in a 500-mL shaking flask (Sakaguchi flask) at 121°C for 15 min. After inoculation with a piece (1 cm × 1 cm, mm thickness) of the slant culture of Aspergillus sp. (FARM AP-21280), the flasks were incubated on a rotary shaker (120 rpm) at 25°C. Culture broth (5 mL) was withdrawn at intervals of 0, 2, 4, 6, 9, 12, 15, 17, 19, and 21 days, and centrifuged at 11,000 × g for 15 min. The supernatant was then passed through a membrane filter (0.45 µm, Merck Millipore Ltd., Darmstadt, Germany) and subjected to high performance liquid chromatography (HPLC) analysis.
Sample preparation for the determination of teadenols A and B and total polyphenol contents in commercial teas Commercial tea (0.5 g) was extracted with 80% EtOH (30 mL) at room temperature for 8 h. After filtration through filter paper (Advantec No. 2), the residual cake was re-suspended in 80% EtOH (30 mL) and extracted in the same manner. After filtration, the filtrates were combined and concentrated using a rotary evaporator.
HPLC analysis of the teadenols HPLC analysis was performed according to the method of Wulandari et al. (2011b). The analytical HPLC system consisted of a PU-2089 HPLC pump (JASCO Corp., Hachioji, Japan) and UV-2075 UV/Vis detector (JASCO). The separation was carried out on a TSKgel ODS-80Ts column (Tosoh Corp., Tokyo, Japan) (250 mm × 4.6 mm i.d., 5 µm) at ambient temperature. The flow rate and injection volume were 0.6 mL/min and 50 µL, respectively. The mobile phase consisted of 1% (v/v) acetic acid in water (A) and CH3CN (B). The gradient conditions were as follows: 90% A at 0 min to 20% A and 80% B at 30 min in a linear gradient elution mode. The teadenols in the eluate were detected at 280 nm. The analytical data were evaluated using the Chromato-PRO software (Runtime Corporation, Sagamihara, Japan).
Measurement of total polyphenols The total polyphenol contents were measured using the Folin-Ciocalteu method, as described previously (Singleton et al., 1999). Briefly, Folin-Ciocalteu reagent (Sigma-Aldrich, St. Louis, MO) was diluted ten-fold with water, and 2.5 mL of this reagent was mixed with 500 µL of each sample. Then, sodium carbonate solution (2.0 mL, 75 g/L) was added. The mixture was incubated at room temperature for 2 h, and the absorbance at 760 nm was measured on a UV-1200 spectrophotometer (Shimadzu, Kyoto, Japan). The readings were compared to a standard curve using gallic acid (Sigma-Aldrich), and the total polyphenolic contents of the samples were expressed as mg gallic acid equivalents (GAE) per mL. All the samples were analyzed in triplicate.
Preparation of teadenols from Aspergillus sp. culture filtrate To obtain the purified teadenols, Aspergillus sp. (FARM AP-21280) was cultivated in the basal medium containing 5.0% EGCG and 1.0% tea powder in 12 culture flasks, as described above. After 19 days, the mycelium was removed by filtration through Advantec No. 2 filter paper. The filtrate (970 mL) was concentrated with a rotary evaporator at 40°C under vacuum to 95 mL. The concentrate was applied to a Sephadex LH-20 column (3.5 × 44 cm, GE Healthcare, Little Chalfont, UK) pre-equilibrated with water. After the column was washed with water (500 mL), the compounds were eluted with 20 – 100% EtOH (20% stepwise gradient, each 400 mL) to give five fractions (F1–F5). Each fraction was concentrated with a rotary evaporator at 40°C under vacuum. Fractions F3 (eluted with 60% EtOH) and F5 (eluted with 100% EtOH) were further purified by preparative HPLC equipped with an InertSustain™ C18 column (10 × 250 mm, 5 µm, GL Sciences, Tokyo, Japan). The concentrated samples (700 µL) were directly injected onto the column. The mobile phase consisted of 1% (v/v) acetic acid in water (A) and CH3CN (B). Elution was carried out with the mobile phase delivered at 3 mL/min according to the following gradient mode: 90% A and 10% B at 0 min, linear gradient to 45% A and 55% B in 17 min, linear gradient to 43% A and 57% B in 13 min, linear gradient to 20% A and 80% B in 10 min, linear gradient to 90% A and 10% B in 10 min. Finally, the initial conditions were held for 10 min. The eluate from the column was fractionated in 3-mL volumes.
NMR spectroscopy 13C and 1H NMR spectra were recorded with a Bruker AVANCE 600 instrument (Billerica, MA, USA). Samples were dissolved in DMSO-d6 containing 0.03% tetramethylsilane (TMS). The chemical shifts are expressed in parts per million (ppm) relative to TMS as an internal standard.
Production of teadenols on the modified Czapek-Dox catechin-containing medium According to the previous study by Wulandari et al. (2011c), EGCG solution was used without the addition of other nutrients for the production of the teadenols by Aspergillus sp. However, we did not observe fungal growth and could not detect either teadenol A or B in the culture broth (data not shown). These results suggested that the EGCG solution was insufficient for fungal growth or teadenols production. Thus, a nutrient medium, the modified Czapek-Dox medium, was used as the basal medium for the production of teadenols by Aspergillus sp. (FARM AP-21280). The Czapek-Dox medium is a semi-synthetic medium that has been generally used for the cultivation of fungi (Raper and Fennell, 1965). The sucrose content was decreased to 0.5%, although the original Czapek-Dox medium contains 3% sucrose as the sole source of carbon. This is because Aspergillus produces citric acid from carbon sources (Xu et al., 1989). The acidity of the basal medium was increased to pH 6.0 because catechins are unstable under alkaline conditions, whereas they are stable under acidic conditions (Zhong et al., 2008; Xie et al., 2013). Additionally, EGCG, the most abundant catechin component (>50%) of tea leaves (Wang and Helliwell, 2000; Xie et al., 2013), was used as a starting material. This ensured that GCG was present in the medium, as a portion of the EGCG was converted into GCG, the C-2 epimer of EGCG, by epimerization during sterilization of the medium by autoclaving (Ikeda et al., 2003; Huang et al., 2004).
Aspergillus sp. (FARM AP-21280) was inoculated to the modified Czapek-Dox medium containing EGCG and GCG, and incubated at 25°C. Then, EGCG, GCG, and teadenols A and B were measured using HPLC. In the initial culture stages (0–4 days), the EGCG and GCG contents in the culture broth were rapidly decreased by the microorganism, regardless of the initial catechin content in the medium. After 6 days, teadenols A and B were detected in the culture broth; the contents of both compounds gradually increased with fermentation, reaching a maximum at 19 days. Regardless of the initial EGCG concentration in the nutrient medium, the maximum level of teadenols production was detected at 19 days, and decreased thereafter. The maximum yields of teadenols by submerged culture are shown in Table 1. Teadenol A was produced at 5.41 ± 1.29 µg/mL, 0.22 ± 0.02 mg/mL, 0.61 ± 0.04 mg/mL, 1.25 ± 0.33 mg/mL, and 1.57 ± 0.08 mg/mL in Czapek-Dox medium containing 0.1%, 1.0%, 2.0%, 5.0%, and 7.0% EGCG, respectively. Teadenol B was produced at 30.9 ± 4.17 µg/mL, 0.50 ± 0.09 mg/mL, 0.93 ± 0.10 mg/mL, 1.60 ± 0.51 mg/mL, and 1.77 ± 0.25 mg/mL in Czapek-Dox medium containing 0.1%, 1.0%, 2.0%, 5.0%, and 7.0% EGCG, respectively. The results show that the maximum contents of teadenols A and B increase in an EGCG dose-dependent manner, although the conversion ratios of the teadenols in the mediums containing 5.0% EGCG are higher than those in the mediums containing 7.0% EGCG. Thus, the addition of 5.0% EGCG in the basal medium is more effective for the production of teadenols; thus, this composition was used for further experiments.
| Teadenol A | Teadenol B | ||||
|---|---|---|---|---|---|
| EGCG (%) | Culture time (days) | Contents | Conversion (%) | Contents | Conversion (%) |
| 0.1 | 19 | 5.41 ± 1.29 a) | 1.13 | 30.9 ± 4.17 a) | 7.88 |
| 1.0 | 19 | 0.22 ± 0.02 b) | 2.46 | 0.50 ± 0.09 b) | 17.7 |
| 2.0 | 19 | 0.61 ± 0.04 b) | 3.07 | 0.93 ± 0.10 b) | 18.5 |
| 5.0 | 19 | 1.25 ± 0.33 b) | 3.14 | 1.60 ± 0.51 b) | 18.8 |
| 7.0 | 19 | 1.57 ± 0.08 b) | 2.62 | 1.77 ± 0.25 b) | 13.1 |
The data represent means ± SD in triplicate.
Effects of green tea powder on the production of teadenols To improve the production of teadenols A and B during submerged culture, commercial green tea powder was added to the medium containing 5.0% EGCG; the results are shown in Table 2. The addition of green tea powder to the medium containing 5.0% EGCG enhanced the teadenol production. The contents of teadenol A produced in the mediums containing 0.5%, 1.0%, 1.5%, and 2.0% tea powder are 2.54 ± 0.41, 3.22 ± 0.23, 2.85 ± 0.40, and 2.65 ± 0.06 mg/mL, respectively. Teadenol B was produced at 3.05 ± 0.31, 4.49 ± 0.38, 3.73 ± 0.57, and 3.70 ± 0.27 mg/mL in the mediums containing 0.5%, 1.0%, 1.5%, and 2.0% green tea powder, respectively. The maximum amounts of the desired products are observed in the medium containing 1.0% green tea powder: the contents of teadenols A and B are 2.6 and 2.8 times higher, respectively, than those in the medium without the green tea powder. In contrast, the teadenols contents in the culture filtrate of Aspergillus sp. grown on the medium containing 1.0% green tea powder without EGCG are relatively low. These results suggest that an initial EGCG content is more effective for the production of teadenols.
| Teadenol A | Teadenol B | ||||||
|---|---|---|---|---|---|---|---|
| EGCG (%) | Tea powder (%) | Culture time (days) | Contents | Conversion (%) | Culture time (days) | Contents | Conversion (%) |
| 0 | 1.0 | 6 | 0.78 ± 0.36 a) | 0.43 | N.D* | N.D* | N.D* |
| 2.0 | 2 | 1.88 ± 0.63 a) | 0.38 | N.D* | N.D* | N.D* | |
| 5.0 | 0 | 19 | 1.25 ± 0.33 b) | 3.14 | 19 | 1.60 ± 0.51 b) | 18.8 |
| 0.5 | 19 | 2.54 ± 0.41 b) | 5.61 | 19 | 3.05 ± 0.31 b) | 34.3 | |
| 1.0 | 19 | 3.22 ± 0.23 b) | 7.12 | 19 | 4.49 ± 0.38 b) | 60.4 | |
| 1.5 | 19 | 2.85 ± 0.40 b) | 6.96 | 19 | 3.73 ± 0.57 b) | 42.9 | |
| 2.0 | 19 | 2.65 ± 0.06 b) | 5.61 | 19 | 3.70 ± 0.27 b) | 41.7 | |
The data representmeans ± SD in triplicate.
As a representative example, the time course of the submerged culture of Aspergillus sp. on the modified Czapek-Dox medium containing 5.0% EGCG and 1.0% green tea powder is shown in Fig. 1. Although the production of the teadenols was enhanced by the addition of green tea powder, the pattern of the time course was not changed from the experiment without green tea powder. A similar effect was reported for the production of Pu-erh tea: during its microbial processing, the moisture content is generally controlled by the addition of water. By using an extract of fresh tea leaves instead of water, the production of metabolites, especially γ-aminobutyric acid (GABA), was enhanced (Hou et al., 2009a). These results suggest that green tea leaves contain compounds that stimulate the expression and/or the activity of enzymes related to teadenol production. Then, these enzymes produce teadenols from EGCG and GCG.
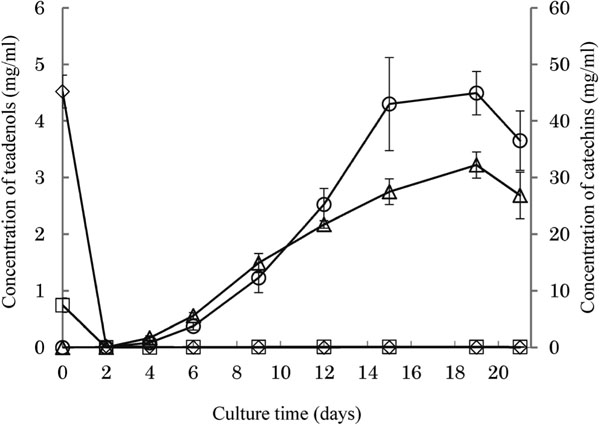
Time course for the production of teadenols A and B in modified Czapek-Dox medium containing 5.0% EGCG and 1.0% green tea powder: ◊, EGCG; □, GCG; △, teadenol A; ○, teadenol B. The data represent means ± SD in triplicate.
The contents of the teadenols and polyphenols in the culture filtrate were compared with those in commercially available microbially fermented teas (Table 3). Teadenols were extracted with 80% ethanol from three kinds of microbially fermented teas (YN fermented with Aspergillus sp.) and Pu-erh teas manufactured in Japan from tea leaves harvested in China (designated as PTC) or Japan (designated as PTJ)), and analyzed by HPLC and the Folin-Ciocalteu method. In the culture filtrate, the polyphenols content is 8.09 ± 0.06 mg GAE/mL, and the teadenols A and B contents are 3.22 ± 0.23 and 4.49 ± 0.38 mg/mL, respectively. These results suggest that the principal components of the polyphenols in the filtrate are teadenols. In contrast, the polyphenols content of the YN extract is 1.21 ± 0.03 mg GAE/mL, and the teadenols A and B contents are 3.10 ± 2.52 and 9.51 ± 4.82 µg/mL, respectively. The ratio of teadenols to polyphenols in the extract of YN is 1%. Thus, the teadenols content in the filtrate is substantially higher than that in the YN extract. Among microbially fermented teas, teadenols A and B are detected in PTJ, but not in PTC. These results are in agreement with previous reports (Wulandari et al., 2011a; Ishimaru et al., 2012a; Ishimaru et al., 2012b). As the fermentation period for Pu-erh tea is 2 months or longer, many compounds are produced from the tea leaves' components by various microorganisms (Jiang et al., 2011; Yamazaki et al., 2013). Some of these products are further converted or decomposed by microbial action during the long fermentation process. The fate of the teadenols is similar to that of such compounds, and their concentrations in commercial post-fermented teas depend on the conditions used during the fermentation of the tea leaves.
| Total polyphenol (mg GAE/mL) | Teadenol A | Teadenol B | ||
|---|---|---|---|---|
| Culture filtrate a) | 8.09 ± 0.06 | 3.22 ± 0.23 d) | 4.49 ± 0.38 d) | |
| Extract | YN | 1.21 ± 0.03 | 3.10 ± 2.52 e) | 9.51 ± 4.82 e) |
| PTCb) | 0.16 ± 0.01 | N.D * | N.D * | |
| PTJc) | 0.53 ± 0.01 | 0.20 ± 0.15 e) | 0.57 ± 0.29 e) |
The data representmeans ± SD in triplicate.
The HPLC profiles for the determination of the teadenols in the culture filtrate and the YN extract are shown in Fig. 2. Two major peaks corresponding to teadenols A and B are observed in the chromatogram of the culture filtrate (B in Fig. 2), and the total area of the minor peaks is considerably smaller than the major peaks. Thus, teadenols A and B are the major products from the submerged culture with the modified Czapek-Dox medium containing 5.0% EGCG and 1.0% green tea powder. Compared with the culture filtrate, the chromatogram of the extract of the commercial post-fermented tea, YN, affords many other peaks besides those due to the pair of teadenols (A in Fig. 2). Furthermore, the total area of the teadenols peaks is considerably lower than those due to other species. These data also support the findings shown in Table 3.

HPLC profiles of extracts of commercial post-fermented Yamabuki-Nadeshiko tea (A) and the culture filtrate of Aspergillus sp. grown on the modified Czapek-Dox medium containing 5.0% EGCG and 1.0% green tea powder (B).
Isolation and identification of teadenols A and B To obtain the purified teadenols, Aspergillus sp. (FARM AP-21280) was cultivated in 12 culture flasks for 19 days. The resultant combined culture filtrates (970 mL) containing 2.73 g teadenol A and 3.15 g teadenol B were subjected to chromatographic separation. A flow chart depicting the procedure for the separation of the teadenols is summarized in Fig. 3. One-third of the culture filtrate (0.91 g teadenol A and 1.05 g teadenol B) was applied to a Sephadex LH-20 column pre-equilibrated with water, and the teadenols adsorbed on the column were eluted with a stepwise gradient of 20 – 100% EtOH. Teadenols A and B were thus separated, eluting with 60% and 100% EtOH, respectively. The purities of both compounds in the corresponding fractions were over 90%, and each fraction was further purified by preparative HPLC using a semi-preparative InertSustain™ C18 column. After HPLC purification, 0.27 g teadenol A and 0.45 g teadenol B were obtained (29.7% and 42.9% recoveries, respectively). In a previous report, a YN extract was applied to a Diaion HP 20 column to remove a large amount of polyphenols other than the teadenols, and the eluate was further fractionated on a Sephadex LH-20 column (Ishimaru et al., 2012b). In contrast, in this study, the teadenols in the filtrate could be directly separated by Sephadex LH-20 chromatography, because the filtrate contained only minor amounts of other compounds. The content of teadenol A in YN was calculated as 0.20 ± 0.15 mg/g dry leaf on the basis of the results indicated in Table 3. The filtrate contained 0.91 g teadenol A (Fig. 3), approximately equivalent to that in 4.5 kg YN.

A schematic diagram of the isolation of teadenols A and B.
The structures of teadenols A and B were analyzed by 1H and 13C NMR, and the data are presented in Tables 4 and 5, respectively. The 1H and 13C NMR spectral data are similar for teadenols A and B, and agree well with previously reported data (Wulandari et al., 2011b). Based on this comparison, the structures of the two purified compounds are confirmed.
| Pos. | The present study | Wulandari et al. (2011b) | ||
|---|---|---|---|---|
| δH | δC | δH | δC | |
| 2 | 4.58 (1H, s) | 70.8 | 4.56 (1H, s) | 70.9 |
| 3 | 4.38 (1H, m) | 70.7 | 4.36 (1H, m) | 70.8 |
| 4 | 2.76 (2H, m) | 24.0 | 2.76 (2H, m) | 24.1 |
| 5 | - | 156.2 | - | 156.3 |
| 6 | 5.92 (1H, d, J = 2.5 Hz) | 95.4 | 5.91 (1H, d, J=2.3 Hz) | 95.6 |
| 7 | - | 156.4 | - | 156.6 |
| 8 | 5.65 (1H, d, J = 2.5 Hz) | 94.1 | 5.64 (1H, d, J=2.3 Hz) | 94.2 |
| 9 | - | 154.6 | - | 154.7 |
| 10 | - | 96.7 | - | 96.9 |
| 11 | - | - | - | - |
| 12 | - | 144.3 | - | 144.1 |
| 13 | 6.53 (1H, d, J = 0.8 Hz) | 110.2 | 6.51 (1H, d, J= 0.9 Hz) | 110.2 |
| 14 | - | 136.4 | - | 136.5 |
| 15 | 5.28 (1H, s) | 118.0 | 5.27 (1H, brs) | 118.0 |
| 5.41 (1H, s) | 5.39 (1H, brs) | |||
| 16 | - | 163.1 | - | 163.2 |
| 5-OH | 9.05 (1H) | 9.00 (1H) | ||
| 7-OH | 9.39 (1H) | 9.33 (1H) | ||
| Pos. | The present study a) | Wulandari et al. (2011b) b) | ||
|---|---|---|---|---|
| δH | δC | δH | δC | |
| 2 | 4.43 (1H, d, J = 10.0 Hz) | 70.9 | 4.36 (1H, d, J=10.5 Hz) | 73.2 |
| 3 | 4.01 (1H, m) | 72.7 | 4.01 (1H, m) | 75.0 |
| 4 | 2.51 (1H, dd, J = 10.5, 15.9 Hz) | 25.6 | 2.66 (1H, dd, J=10.3, 15.7 Hz) | 27.1 |
| 3.03 (1H, dd, J = 6.2, 15.9 Hz) | 3.20 (1H, dd, J=5.9, 15.7 Hz) | - | ||
| 5 | - | 156.2 | - | 157.7 |
| 6 | 5.98 (1H, d, J = 2.5 Hz) | 95.8 | 5.96 (1H, d, J=2.3 Hz) | 96.9 |
| 7 | - | 156.8 | - | 158.2 |
| 8 | 5.82 (1H, d, J = 2.5 Hz) | 94.0 | 5.91 (1H, d, J=2.3 Hz) | 95.6 |
| 9 | - | 154.1 | - | 156.0 |
| 10 | - | 97.9 | - | 100.0 |
| 11 | - | - | - | - |
| 12 | - | 143.3 | - | 145.0 |
| 13 | 6.59 (1H, s) | 111.5 | 6.63 (1H, s) | 113.2 |
| 14 | - | 136.5 | - | 138.6 |
| 15 | 5.29 (1H, s) | 112.5 | 5.33 (1H, s) | 113.3 |
| 5.40 (1H, s) | 5.51 (1H, s) | - | ||
| 16 | - | 162.9 | - | 166.0 |
| 5-OH | 9.17 (1H) | - | ||
| 7-OH | 9.48 (1H) | - | ||
Fermentation is an invaluable technology with great potential for the production or extraction of compounds from natural sources (Hur et al., 2014). New bioactive compounds can be discovered after fermentation, such as the teadenols, which are expected to function as new anti-metabolic syndrome compounds (Yanagita et al., 2011). Moreover, the constituents in the fermented products can be altered by modification of the fermentation process. Therefore, the culture conditions for the production of teadenols A and B using Aspergillus sp. (FARM AP-21280) were studied herein.
Aspergillus sp. (FARM AP-21280) produced teadenols as the major products in the modified Czapek-Dox medium containing EGCG and GCG, and the teadenols contents increased depending on the initial EGCG concentration in the medium. However, the conversion ratio of the teadenols from EGCG decreased for EGCG concentrations exceeding 5.0%. The addition of green tea powder to the medium improved the production of the teadenols. The optimum concentrations of EGCG and green tea powder for the production of teadenols were 5.0% and 1.0%, respectively. The culture filtrate contained 3.22 ± 0.23 mg/mL teadenol A and 4.49 ± 0.38 mg/mL teadenol B, and the contents of polyphenols other than teadenols were extremely low.
Teadenols A and B in the culture filtrate could be separated by Sephadex LH-20 column chromatography with good resolution, because the culture filtrate contained mainly the teadenols with few impurities. The 1H and 13C NMR spectral data for teadenols A and B corresponded reasonably well with the published data (Wulandari et al., 2011b).
This combined process for the production of teadenols, comprising the submerged culture of Aspergillus sp. (FARM AP-21280) in the modified Czapek-Dox medium containing 5.0% EGCG and 1.0% green tea powder and sequential chromatographic separation on Sephadex LH-20 and InertSustain™ C18 columns, showed adequate efficiency. This procedure may solve the problem of teadenol supply for their use as food additives. Further studies to elucidate the enzymes related to the biosynthesis of teadenols are now in progress.
Acknowledgements The authors acknowledge Dr.Denbei Kawamura of Riverson Co., Ltd. for providing the Aspergillus sp. (FARM AP-21280) used in these experiments.
(-)-epigallocatechin 3-O-gallate
GCGgallocatechin 3-O-gallate
GAEgallic acid equivalents
PDApotato dextrose agar
PTCPu-erh tea manufactured from tea leaves harvested in China
PTJPu-erh tea manufactured from tea leaves harvested in Japan
TMStetramethylsilane
YNYamabuki-Nadeshiko tea