2017 年 23 巻 2 号 p. 249-254
2017 年 23 巻 2 号 p. 249-254
Fewer studies on the effects of heat treatment on casein under acidic conditions have been reported compared with those under neutral conditions. This study aims to clarify the effect of heat treatment under acidic conditions on the properties and functions of milk proteins. The turbidity of reconstituted skimmed milk increased with increasing temperature (70 – 90°C) and pH (3.40 – 3.55). Suspensions of heated samples were stable and did not sediment at low-speed centrifugation. Mean particle diameters of heat-induced particles were 200 – 680 nm. Sodium dodecyl sulfate–polyacrylamide gel electrophoresis and ion-exchange high-performance liquid chromatography revealed that heat-induced colloidal particles comprised αS1-casein and β-casein. αS2-Casein did not participate in aggregate formation. The proportion of αS1-casein and β-casein did not change regardless of temperature, whereas the proportion of β-casein was dependent upon pH. The results showed that heat-sensitivity of casein constituents at pH 3.30 – 3.55 is markedly different from that under neutral conditions, and some caseins aggregate into stable colloidal particles upon heating.
Milk and milk proteins are important materials for processing in the food industry. Understanding the properties and functions of milk and milk protein constituents is important for their use as ingredients in food processing. Milk and milk proteins are used for the manufacture of sour milk beverages, and in some cases, these are pasteurized or sterilized by heating.
In bovine milk, caseins are aggregated into approximately spherical colloidal particles with a diameter of 20 – 600 nm termed “casein micelles”. They contain four types of casein molecules at a molar ratio of αS1:αS2:β:κ = 4:1:4:1.3, and 6.6% of inorganic materials (calcium phosphate). Calcium phosphate crosslinks casein molecules by their phosphate groups in casein micelles (Aoki et al., 1986a, 1987), and is termed as “colloidal calcium phosphate” or “micellar calcium phosphate” (MCP).
Casein micelles in milk constitute remarkably stable systems that can withstand rigorous conditions commonly associated with commercial dairy processing (Fox, 1982). However, under certain conditions of temperature and pH, the structures of casein micelles can be disrupted, resulting in altered functional properties. The structures of casein micelles are closely associated with their functional properties. Accordingly, the effects of heat treatment and pH on their structure and functional properties have been reported by many researchers (Walstra, 1990; Anema and Klostermeyer, 1997a). Heating milk at >110°C has been shown to cause a substantial increase in the level of soluble casein, of which approximately 40% is κ-casein (Aoki et al., 1974, 1975). Subsequent studies (Singh and Fox, 1985, 1986; Singh and Creamer, 1991) confirmed that the dissociation of κ-casein from casein micelles is pH-dependent upon heating milk at >90°C. Subsequently, Anema and Klostermeyer (1997b) reported that, at pH ≥6.8, marked dissociation of αS-casein and β-casein from casein micelles occurs upon heating at <100°C.
In those studies, milk samples were heated at neutral conditions. The conformation of casein under acidic conditions may be different from that under neutral conditions. Casein under acidic conditions does not take the form of micellar particles crosslinked by MCP because the latter dissociates from casein micelles. Casein precipitates at its isoelectric point and becomes more soluble as pH decreases further. In contrast to studies on the effects of heat treatment upon casein under neutral conditions, fewer studies on the effects of heat treatment upon casein at a lower pH than the isoelectric point of casein have been reported. Accordingly, in the present study, we focused on clarifying the effects of heat treatment under acidic conditions on the properties and functions of casein. We found that, at pH 3.30 – 3.55, some casein molecules aggregated into colloidal particles upon heating.
Milk Supply (Sample) Medium-heat skimmed milk powder (the nitrogen index of whey protein is 3.10; Sanderson, 1970) and raw skimmed milk were obtained from Minami Nippon Dairy CO-OP Co., Ltd. (Miyazaki, Japan). Experimental samples of skimmed milk were prepared to provide 0.35% (w/w) of protein in Milli-Q water (Millipore, Bedford, MA, USA). A sample of reconstituted skimmed milk was allowed to equilibrate at room temperature with gentle stirring for 2 h before further treatment.
Adjustment of pH and Heat Treatments pH values of skimmed-milk samples were adjusted by rapid addition of 1 M HCl down to pH 3.60 to avoid precipitation at the isoelectric point, and then adjusted by slow addition of 0.1 M HCl to the prescribed pH value. pH-adjusted solutions were allowed to equilibrate at room temperature with gentle stirring for 2 h. Subsamples (3 mL) at each pH were transferred to test tubes and heated for 15 min in a thermostatically controlled water-bath pre-set to 20 – 90°C. After heat treatment, samples were cooled to room temperature by immersion of test tubes in cold running water.
Turbidity Measurement and Mean Particle Diameter To each 3-mL heated sample, 7 mL of Milli-Q water was added, followed by measurement of absorbance at 600 nm using quartz cells (10 mm) and a U-3010 spectrophotometer (Hitachi, Tokyo, Japan). The mean value of triplicate turbidity measurements was recorded. The mean particle diameter of heated samples was determined using a laser scattering particle size distribution analyzer (LA-920; Horiba, Kyoto, Japan). The diluted solution used was 10 mM citrate buffer (pH 3.45).
Protein and Mineral Contents Soluble protein, calcium, and inorganic phosphate (Pi) were defined as those in the supernatant after centrifugation at 20,000 × g for 10 min at 20°C. For determination of Pi, the supernatant underwent ultrafiltration through Vivaspin 4 (molecular mass cut-off = 10,000; Vivascience, Göttingen, Germany). Phosphorus content in the ultrafiltrate was considered as Pi. The contents of calcium and phosphorous were determined using an inductively coupled plasma–atomic emission spectrometer (ICPS-7510; Shimadzu, Kyoto, Japan) after digestion with HNO3 using a microwave digestion system (Ethos 1; Milestone, Sorisole, Italy). The protein content was determined using the micro-Kjeldahl method.
Electrophoresis Heat-induced particle and soluble protein fractions were obtained as sediment and supernatant, respectively, after centrifugation of heated samples at 20,000 × g for 10 min at 20°C. Individual proteins in heat-induced particle and soluble protein fractions were determined by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) using 12.0% gels under reducing and non-reducing conditions in the presence and absence of 2-mercaptoethanol, respectively, according to the method of Laemmli (1970). Heat-induced particles were washed thrice with purified water. The heat-induced particles were dissolved in buffer to give a protein concentration of 0.12%. The control (non-heated sample) and soluble protein fractions were diluted thrice with the sample buffer. For SDS-PAGE under non-reducing conditions, samples were heated at 45°C for 5 min in a block heater. For reducing conditions, 5% of 2-mercaptoethanol was added to samples followed by heating at 95°C for 10 min in a block heater. Samples (10 µL) were then loaded onto the SDS gel, and the latter was then run in a Mini-PROTEAN® system (Bio-Rad Laboratories, Hercules, CA, USA) at 200 V using a power-supply unit (PowerPac HC; Bio-Rad Laboratories). Protein bands were fixed and stained using a solution of Coomassie Blue G-250. After destaining, proteins were identified by comparison with the molecular weight standard proteins obtained from Bio-Rad Laboratories.
High-Performance Ion-Exchange Chromatography (IE–HPLC) IE–HPLC was conducted using an Agilent 1100 (Agilent Technologies, Santa Clara, CA, USA) equipped with a TSKgel® DEAE-5PW column (7.5 mm × 75 mm; Tosoh Bioscience, Tokyo, Japan) attached to a TSK guard column (6 mm × 10 mm) according to the method of Aoki et al. (1986b). Heated samples were centrifuged at 20,000 × g for 10 min at 20°C. The heat-induced particles were washed thrice with purified water, and then dissolved in imidazole–urea chromatography buffer (pH 8.0; 0.02 M imidazole, 3.3 M urea, 0.08 M NaCl) containing 0.02 M 2-mercaptoethanol and allowed to stand overnight at 5°C. Elution was conducted by a linearly increasing concentration gradient of NaCl at 0.5 mL/min. The NaCl concentration was increased from 0.08 M to 0.3 M for 70 min.
The protein composition in fractions obtained by chromatography was determined by measurement of the peak area combined with individual extinction coefficients at 280 nm. The extinction coefficient E1% (cm−1) at 280 nm was: αS1-casein, 10.5; αS2-casein, 11.0; β-casein, 4.6; and κ-casein, 9.5 (Swaisgood, 1982).
Calculation of Net Proton Charge The net proton charges of casein constituents were calculated from the amino acid compositions of the primary structure (Swaisgood, 1982) using the following formula:
 |
 = net proton charge, (
= net proton charge, ( )max = maximum net proton charge, n = number of dissociable groups, Ki = dissociation constant of a dissociating group, and aH = hydrogen ion activity (this was regarded as hydrogen ion concentration). The apparent pKi values were: α-carboxyl, 3.6; phosphoseryl, pK1 = 1.5, pK2 = 6.4; β, γ-carboxyl, 4.9; histidyl, 6.6; α-amino, 7.4; phenolic, 9.8; ε-amino, 10.0; and guanidyl, 12.0.
)max = maximum net proton charge, n = number of dissociable groups, Ki = dissociation constant of a dissociating group, and aH = hydrogen ion activity (this was regarded as hydrogen ion concentration). The apparent pKi values were: α-carboxyl, 3.6; phosphoseryl, pK1 = 1.5, pK2 = 6.4; β, γ-carboxyl, 4.9; histidyl, 6.6; α-amino, 7.4; phenolic, 9.8; ε-amino, 10.0; and guanidyl, 12.0.
The effect of pH on the turbidity of reconstituted skimmed milk heated at 20 – 90°C for 15 min is shown in Figure 1A. No change in turbidity was observed at 20 – 70°C at each pH value. However, at a certain temperature (>75°C), turbidity increased markedly and linearly with increasing temperature, and increased as the pH was increased. The turbidity of heated raw skimmed milk showed a similar tendency to that of reconstituted skimmed milk except for samples adjusted to pH values of 3.30 and 3.35 (Figure 1B). The turbidities of reconstituted skimmed milk adjusted to pH values of 3.30 and 3.35 were lower than those of raw skimmed milk. These results suggested that heat treatment during the manufacture of skimmed milk powder affected the turbidity of reconstituted skimmed milk at pH < 3.35.

Effect of temperature and pH on the turbidity of (A) reconstituted skimmed milk and (B) raw skimmed milk. Skimmed-milk samples (3 mL) were heated for 15 min at each temperature, then 7 mL of Milli-Q water was added, followed by measurement of absorbance at 600 nm. ○, pH 3.55; ●, pH 3.50; □, pH 3.45; ■, pH 3.40; △, pH 3.35; ▴, pH 3.30. Data show the mean values of three determinations with standard deviation < 0.02.
Suspensions of heated samples were stable and did not sediment at low-speed centrifugation. Mean particle diameters of heat-induced particles in reconstituted skimmed milk were 200 – 680 nm (Figure 2). The turbidity of a colloidal suspension is related to the size and scattering properties of dispersed particles (Rajput et al., 1983; Creamer, 1985). The mean particle diameters of heat-induced particles in reconstituted skimmed milk increased with increasing pH and temperature in accordance with an increase in turbidity.

Effect of temperature and pH on the mean particle diameter of heat-induced particles in reconstituted skimmed milk. ○, pH 3.55; ●, pH 3.50; □, pH 3.45; ■, pH 3.40. Data show the mean values of three determinations with standard deviation < 0.05.
The percentage change in the amount of soluble protein that did not precipitate by centrifugation at 20,000 × g in heated samples relative to an unheated sample was determined. The amount of soluble protein decreased at a certain temperature, and decreased as the pH was increased (Figure 3). In other words, the protein in heat-induced particles obtained by centrifugation increased with increasing pH and temperature. The increase in turbidity was likely to be due to the increased volume and increased number of colloidal particles. The contents of soluble calcium and Pi in supernatants after centrifugation were not affected by pH and temperature (data not shown). Casein under acidic conditions does not take the form of micellar particles crosslinked by MCP, but is soluble at pH 3.30 – 3.55. Calcium and Pi in reconstituted skimmed milk at pH 3.30 – 3.55 did not appear to be involved in the composition of heat-induced colloidal particles because calcium and Pi were not bound to heat-induced colloidal particles. The effect of heating on turbidity was strongly dependent on pH. At a temperature of >75°C, turbidity increased markedly and linearly with increasing temperature. These results suggest that an increase in turbidity induced by heating may be due to partial aggregation of caseins. Heat-induced aggregation of caseins increased with increasing pH leading to a decrease in the net charge on caseins at a pH below the isoelectric point.
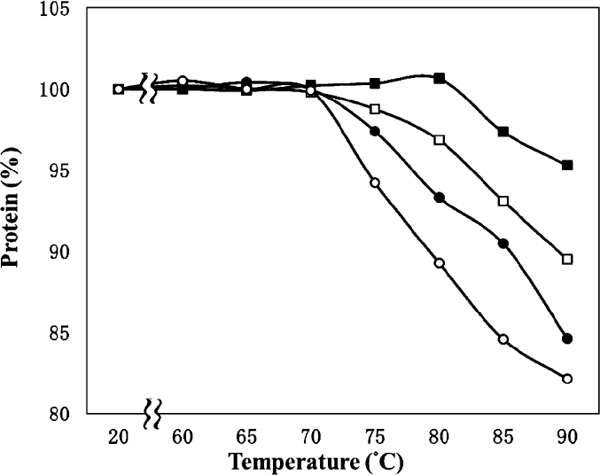
Effect of temperature and pH on soluble protein in reconstituted skimmed milk. ○, pH 3.55; ●, pH 3.50; □, pH 3.45; ■, pH 3.40. Results are shown as percentage change relative to the unheated sample at each pH value. Data show the mean value of three determinations with standard deviation < 0.02.
Heat-induced colloidal particles obtained from reconstituted skimmed milk were confirmed by SDS–PAGE in the presence of 2-mercaptoethanol, and consisted mainly of αS-casein and β-casein (Figure 4). κ-Casein and whey protein were observed only in the soluble protein fraction. Bands of κ-CN, β-lactoglobulin (β-lg), and α-lactalbumin (α-la) were observed in the presence of 2-mercaptoethanol, whereas the intensities of the bands of these proteins decreased in the absence of 2-mercaptoethanol (data not shown), suggesting that these proteins are present as complexes formed by intermolecular disulfide linkages. The complex of κ-casein with whey protein seems to be formed during the manufacture of skimmed milk powder (Guyomarc'h et al., 2003). κ-Casein might prevent precipitation of β-lg and α-la by formation of complexes, and the complexes did not precipitate by centrifugation at 20,000 × g for 10 min. No difference was observed in the SDS-PAGE patterns of the sediment in the presence and absence of 2-mercaptoethanol. It appears that heat-induced aggregation may primarily be due to an increase in hydrophobic interactions between αS-casein and β-casein.

Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) patterns of heat-induced particle and soluble protein obtained from reconstituted skimmed milk under reducing conditions. Line 1: marker protein; line 2: reconstituted skimmed milk (unheated); line 3 – 6: heat-induced particle obtained from reconstituted skimmed milk heated at 85°C at pH 3.40, 3.45, 3.50, and 3.55, respectively; line 7 – 10: soluble protein obtained from reconstituted skimmed milk heated at 85°C at pH 3.40, 3.45, 3.50, and 3.55, respectively. (I) Immunoglobulin-G, lactoferrin, and bovine serum albumin; (II) αs-casein; (III) β-casein; (IV) κ-casein; (V) β-lg; and (VI) α-la.
IE-HPLC was conducted to determine the protein composition of heat-induced colloidal particles. Figure 5 shows the IE-HPLC patterns of proteins in reconstituted skimmed milk before heating and heat-induced colloidal particles obtained by centrifugation. Elution profiles were divided into seven fractions. As reported by Aoki et al. (1986b), fractions 3, 4, 5, 6, and 7 were considered to contain κ-casein, unidentified molecule, β-casein, αS2-casein, and αS1-casein, respectively. γ-Casein and 2-mercaptoethanol were eluted at the retention time of fraction 1. Retention times of early and late peaks eluted in fraction 2 coincided with those of purified β-lg and α-la, respectively. These results suggest that heat-induced colloidal particles are composed of αS1-casein and β-casein. The casein composition was estimated from peak areas combined with individual extinction coefficients at 280 nm. The ratio of αS1-casein and β-casein did not change at pH 3.50 regardless of temperature, whereas the ratio of β-casein increased as pH was increased at 85°C (Table 1).

High performance ion-exchange chromatography (IE-HPLC) patterns of heat-induced particles obtained from reconstituted skimmed milk heated at 85°C under acidic conditions. Column; TSKgel® DEAE-5PW (7.5 mm × 75 mm), flow rate; 0.5 mL/min; elution conditions; see text. (A) Reconstituted skimmed milk (unheated), (B, C, D and E) heat-induced particles obtained from reconstituted skimmed milk heated at 85°C at pH 3.40, 3.45, 3.50, and 3.55, respectively.
| (A) | |||
| Temperature | pH | αS1-Casein | β-Casein |
| 75 | 3.50 | 80.2 | 19.8 |
| 80 | 3.50 | 80.8 | 19.2 |
| 85 | 3.50 | 80.7 | 19.3 |
| 90 | 3.50 | 79.9 | 19.6 |
| (B) | |||
| Temperature | pH | αS1-Casein | β-Casein |
| 85 | 3.40 | 86.9 | 13.1 |
| 85 | 3.45 | 83.9 | 16.1 |
| 85 | 3.50 | 80.7 | 19.3 |
| 85 | 3.55 | 75.6 | 24.4 |
It is noteworthy that αS2-casein did not participate in aggregate formation despite that it is known as the most heat-sensitive casein constituent at neutral pH. Haga et al. (1983) reported that αS2-casein contained approximately 20% α-helix and a small amount of β-sheet, and that the content of the α-helix form in αS2-casein was higher than that in αS1-casein and β-casein. Under neutral conditions, heat treatment of αS2-casein at 90°C for 15 min causes destruction of approximately 35% of the α-helix, and the αS2-casein solution becomes very turbid at >100°C (Haga et al., 1983). Nevertheless, αS2-casein did not participate in aggregate formation in the present study. To determine the reason for the stability of αS2-casein under acidic conditions, the net proton charge from the primary structure was calculated. Calculated values are shown in Table 2. The positive net charges of the αS2-casein family at pH 3.30 – 3.60 were higher than that of αS1-casein and β-casein. In addition to high net charge, αS2-casein is the most hydrophilic of all casein constituents, and its average hydrophobicity is in the range of globular proteins (Swaisgood, 1982). High net charge and hydrophilicity are suggested as the reasons for the stability of αS2-casein under acidic conditions.
| Protein | Net proton charge | |||
|---|---|---|---|---|
| pH | pH | pH | pH | |
| 3.30 | 3.45 | 3.60 | 6.70 | |
| αS1-Casein B-8P | 17.0 | 16.6 | 16.1 | −21.8 |
| αS2-Casein A-10P | 23.1 | 22.7 | 22.2 | −14.0 |
| αS2-Casein A-11P | 22.1 | 21.7 | 21.2 | −15.7 |
| αS2-Casein A-12P | 21.1 | 20.7 | 20.2 | −17.4 |
| αS2-Casein A-13P | 20.2 | 19.7 | 19.2 | −19.1 |
| β-Casein A-5P | 15.2 | 14.9 | 14.5 | −13.0 |
| β-lactoglobulin | 20.0 | 19.7 | 19.2 | −8.5 |
| α-lactalbumin | 16.2 | 16.0 | 15.7 | −3.3 |
It is interesting that the heat-sensitivity of casein constituents under an acidic condition is markedly different from that under a neutral condition. αS1-Casein showed a higher heat-sensitivity than β-casein under an acidic condition (Figure 5, Table 1). However, the positive net charge of αS1-casein was higher than that of β-casein. The average hydrophobicity of αS1-casein is lower than that of β-casein. Casein micelles are recognized to dissociate at a high temperature >100°C. However, Anema and Klostermeyer (1997b) showed that casein dissociates from casein micelles upon increasing the temperature to ≤70°C, whereas the rate of dissociation decreases at higher temperatures. Thus, the properties of casein constituents are affected by heating at a temperature <100°C. This suggests that heating at a temperature <100°C may also cause a conformational change in casein constituents. One reason for the higher heat-sensitivity of αS1-casein under acidic conditions may be a conformational change rather than the net charge, average hydrophobicity, and change of secondary structure.
We found that aggregation of αS1-casein and β-casein occurred at >70°C under acidic conditions. It is well known that no aggregation of caseins occurs when casein solution is heated at near the neutral pH in the absence of calcium ion. Thus, the heat-sensitivity of casein constituents under an acidic condition is markedly different from that under a neutral condition, and the heat-induced aggregate of αS1-casein and β-casein was present as a stable colloidal particle at <90°C. Such aggregation behavior has not previously been reported.