2017 Volume 23 Issue 2 Pages 275-281
2017 Volume 23 Issue 2 Pages 275-281
Turmeric (Curcuma longa) is used as a food ingredient, coloring agent, and traditional medicine. While various medicinal properties of turmeric have long been known, scientific reports on its prevention and alleviation of alcohol hangover are scarce. In this study, we employed an assay system for the preventive effect of a turmeric extract against ethanol-induced hepatocyte injury, a presumed cause of alcohol hangover, and screened for its active compounds. The preventive effect of the turmeric extract and its fractions was assessed using primary hepatocytes isolated from Sprague-Dawley rats. The turmeric extract containing 6 µM curcuminoids showed stronger cytoprotective activity than the purified curcuminoids at 6 µM. The structures of additional active compounds were determined as ar-turmerone and bisacurone by NMR and mass spectrometry. Among curcumin, ar-turmerone, and bisacurone, bisacurone appeared to be most effective and showed preventive effects at concentrations as low as 1 µM.
Turmeric is a zingiberaceous perennial plant native to tropical Asia and its rhizome is used as a food ingredient, coloring agent, and traditional medicine (Khajehdehi, 2012). The characteristic compounds in the rhizome are curcuminoids, yellow-pigments that include curcumin, demethoxycurcumin, and bisdemethoxycurcumin. Turmeric and curcuminoids have been reported to possess a variety of medicinal properties, including anti-oxidant (Balogun et al., 2003), anti-inflammatory (Gupta et al., 2008), anti-cancer (Anand et al., 2008), anti-dementia (Ishrat et al., 2009), and hepatoprotective activities (Sengupta et al., 2011). In Japan, it has recently become widely known that turmeric has beneficial effects in the prevention and alleviation of alcohol hangover. While there are some reports on its effects on alcohol metabolism and on the prevention of ethanol-induced liver damage (Nwozo et al., 2014; Hamano et al., 2007), the scientific literature remains scarce, and its active compounds are not well understood.
Alcoholic beverages play an important role in enhancing the flavor of foods as well as the dining experience. Excessive drinking, however, may cause alcohol hangover. Alcohol hangover is characterized by unpleasant physical symptoms, including headache, nausea, thirst, fatigue, sleepiness, and weakness, which occur after the heavy consumption of alcohol (Penning et al., 2010). After ingestion, alcohol is absorbed from the stomach and small intestine, and subsequently metabolized by the liver. Within the liver cells, alcohol is rapidly metabolized to the more toxic acetaldehyde by alcohol dehydrogenase (ADH), cytochrome P450 (CYP2E1), and catalase (Zakhari, 2006; Cederbaum, 2012). Acetaldehyde is further metabolized by aldehyde dehydrogenase (ALDH) to acetate (Zakhari, 2006; Cederbaum, 2012). Excessive alcohol intake leads to liver damage through reactive oxygen species production, lower cellular antioxidant levels, and aggravation of oxidative stress (Dey and Cederbaum, 2006; Zhou et al., 2001; Masini et al., 1994). Alcohol consumption also increases oxygen use in hepatocytes, causing oxygen deficits, and may ultimately interfere with energy production in the liver, resulting in impairment of the cell's ability to perform critical functions including the repair of alcohol-induced cell injury (Cunningham and Van Horn, 2003). This temporary liver damage may lead to hangover-related symptoms such as fatigue and weakness, which appear when alcohol has disappeared from the body.
In this work, we focused on hepatocyte injury as a cause of alcohol hangover, and in addition to curcuminoids, isolated and identified two active compounds from a turmeric extract using an assay system we designed based on ethanol-induced hepatocyte injury.
Materials Turmeric extract powder prepared by extracting dried rhizomes of turmeric (Curcuma longa) with hot water and spray drying, which contained 4.4% curcuminoids, and a powder of purified curcuminoids comprising curcumin (ca. 75%), demethoxycurcumin (ca. 18%), and bisdemethoxycurcumin (ca. 3%) were obtained from a local supplier. Reagent grade curcumin (> 98%) was purchased from NAGARA Science (Gifu, Japan).
Extraction and isolation The extraction and isolation procedures of the active compounds are shown in Fig. 1. The turmeric extract powder was first extracted with methanol, and the extract was then separated into 10 fractions using silica gel open-column chromatography (silica gel. Wakogel® C-300; Wako Pure Chemical Industries, Osaka, Japan). Fractions 1 to 5 were eluted with n-hexane/ethyl acetate = 10:1 as fractions more hydrophobic than curcuminoids, and fractions 6 to 10 were eluted with chloroform/methanol = 30:1 as fractions more hydrophilic than curcuminoids. Curcuminoids were retained in the column, and the fractions contained little or no curcuminoids. A portion of each fraction was dried, dissolved in an appropriate amount of dimethylsulfoxide, and subjected to a cell viability assay. Fraction 2 was further fractionated by preparative HPLC (LC-10AD; Shimadzu, Tokyo, Japan) with an ODS-3 column (250 mm × 20 mm i.d.; GL Sciences, Tokyo, Japan), using a mobile phase of methanol/water = 9:1 to obtain fractions 2-1 to 2-5. Compound 1 was finally isolated from fraction 2-2 by recycling preparative HPLC (LC-9110NEXT; Japan Analytical Industry, Tokyo, Japan) with an ODS-3 column (250 mm × 20 mm i.d.; GL Sciences) using methanol/water = 9:1. Compound 2 was isolated from fraction 7 by using acetonitrile/water = 1:1 as the mobile phase. 1H- and 13C-nuclear magnetic resonance (NMR) spectra were obtained on a JEOL ECA-500 spectrometer (JEOL, Tokyo, Japan) at 500 and 125 MHz, respectively. Mass spectra were obtained on a Fourier transform mass spectrometer (Orbitrap; Thermo Fisher Scientific, Kanagawa, Japan). Data for the analyses of these 2 compounds by mass spectrometry and NMR are as follows:

Fractionation of active compounds from turmeric extract powder, yields are per 10 g of turmeric extract powder.
Compound 1, FTMS, m/z 217.1584 [M + H]+; 1H-NMR (CDCl3) δ, 7.10 (4H, m), 6.02 (1H, d, JH = 1.2 Hz), 3.28 (1H, ddd, JH = 16.1, 6.9, 6.9 Hz), 2.70 (1H, dd, JH = 15.5, 5.7 Hz), 2.60 (1H, dd, JH = 16.1, 6.9 Hz), 2.30 (3H, s), 2.10 (3H, s), 1.85 (3H, s), 1.23 (3H, d, JH = 6.9 Hz); 13C-NMR (CDCl3) δ, 200.1 (C-9), 155.3 (C-11), 143.8 (C-4), 135.7 (C-1), 129.2 (C-3, C-5), 126.7 (C-2, C-6), 124.2 (C-10), 52.8 (C-8), 35.4 (C-7), 27.8 (C-15), 22.1 (C-13), 21.1 (C-12), 20.8 (C-14).
Compound 2, FTMS, m/z 253.1795 [M + H]+, 275.1618 [M + Na]+, 291.1350 [M + K]+; 1H-NMR (CDCl3) δ, 6.07 (1H, dd, JH = 1.2, 1.2 Hz), 5.64 (2H, s), 3.79 (1H, dd, JH = 7.0, 3.2 Hz), 2.44 (1H, dd, JH = 14.9, 5.2 Hz), 2.30 (1H, m), 2.26 (1H, m), 2.20 (1H, m), 2.14 (3H, d, JH = 1.2 Hz), 1.89 (3H, d, JH = 1.2 Hz), 1.82 (1H, ddd, JH = 10.9, 7.4, 2.8 Hz), 1.71 (1H, ddd, JH = 12.6, 6.3, 6.3 Hz), 1.52 (2H, br s), 1.30 (3H, s), 0.91 (3H, d, JH = 6.7 Hz); 13C-NMR (CDCl3) δ, 200.7 (C-9), 155.7 (C-11), 132.4 (C-2), 132.2 (C-3), 124.1 (C-10), 73.5 (C-5), 70.2 (C-4), 48.8 (C-8), 36.5 (C-1), 33.2 (C-7), 27.9 (C-6), 27.8 (C-13), 24.0 (C-15), 20.9 (C-12), 17.0 (C-14); 1H-NMR (D2O) δ, 6.22 (1H, m), 5.66 (1H, dd, JH = 10.31, 2.3 Hz), 5.52 (1H, ddd, JH = 10.0, 2.6, 1.2 Hz), 3.71 (1H, ddd, JH = 2.9, 2.9, 1.7 Hz), 2.50 (1H, dd, JH = 14.9, 5.7 Hz), 2.24 (1H, dd, JH = 14.6, 8.9 Hz), 2.16 (1H, m), 2.05 (1H, m), 1.99 (3H, d, JH = 1.2 Hz), 1.84 (3 H, d, JH = 1.2 Hz), 1.68 (2H, m), 1.18 (3 H, s), 0.78 (3H, d, JH = 6.9 Hz); 13C-NMR (D2O) δ, 207.36 (C-9), 159.51 (C-11), 132.83 (C-2), 130.98 (C-3), 123.93 (C-10), 72.65 (C-5), 69.82 (C-4), 48.35 (C-8), 35.89 (C-1), 33.59 (C-7), 27.01 (C-6), 26.98 (C-13), 23.71 (C-15), 20.47 (C-12), 15.80 (C-14)
Isolation and culture of primary rat hepatocytes Hepatocytes were isolated from 6-week-old male SD rats (body weight 200 – 230 g; Charles River Japan, Kanagawa, Japan) by the two-step collagenase perfusion method described by Ichihara et al. (Ichihara et al., 1980). All animal experiments were approved by the Ethics Committee for Animal Experiments of House Wellness Foods Corporation and carried out in its facility. The isolated hepatocytes were suspended at a density of 2 × 105 cells/mL in Williams' E medium supplemented with 10% fetal bovine serum (FBS), 0.1 µM insulin, 1 µM dexamethasone, 100 units/mL penicillin, and 100 µg/mL streptomycin, and cultured at 6 × 104 cells/well in a 48-well tissue culture plate (Falcon; Thermo Fisher Scientific, Waltham, MA) pre-coated with collagen type I-C (Nitta Gelatin Inc., Osaka, Japan) in a humidified chamber at 37°C under 5% CO2 and 95% air for 4 h.
Sample treatment and cell viability assay Figure 2 shows the schedule for sample/ethanol treatment and cell viability assay. After 4 h of preculture, hepatocytes were treated with test samples by changing the culture medium to the one with added test samples and without insulin and dexamethasone supplementation. The test samples were dissolved in a small amount (0.1% of the medium volume) of dimethylsulfoxide before being added to the culture medium. After 16 h of treatment with the test samples, the hepatocytes were exposed to ethanol by changing the culture medium to the one containing 1.5 to 3.0% (vol/vol) ethanol and 25 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), with reduced FBS supplementation (1%), and without insulin and dexamethasone supplementation. After 24 h of exposure to ethanol, cell viability was determined by the resazurin assay using a CellQuanti-Blue Cell Viability Assay Kit (BioAssay Systems, Hayward, CA). The fluorescence intensity was measured with a microplate reader (530 nm excitation and 615 nm emission: MTP-800lab, CORONA ELECTRIC, Ibaraki, Japan, or 540 nm excitation and 590 nm emission: Tecan Infinite M200, Grödig, Austria). Viability of cells exposed to ethanol with or without prior treatment with the test samples was calculated as follows:

A schedule for sample/ethanol treatment and cell viability assay.
Cell viability (%) = (F − Fblank) / (Fcontrol − Fblank) × 100, where F is the fluorescence intensity from cells exposed to ethanol with or without prior sample treatment, Fcontrol is the averaged fluorescence intensities from cells without exposure to ethanol, and Fblank is the averaged fluorescence intensity of the blank control. The viability of cells exposed to the same ethanol concentration without prior sample treatment varied widely from experiment to experiment because of the variability in individual animals from which the hepatocytes were prepared. Therefore, for each hepatocyte preparation, we ran assays using various ethanol concentrations ranging from 1.5 to 3.0% (vol/vol) and selected a concentration at which the average viability of cells exposed to ethanol without sample treatment was closest to 50% and used the results from that particular concentration. Further, to minimize variability and to more clearly show sample effects, we normalized the results within each assay by the difference between the average viability of cells unexposed to ethanol (100%) and that of cells exposed to ethanol without sample treatment, and expressed the sample effects in cytoprotective activity as defined by the following equation.
Cytoprotective activity (%) = (V − V0) / (100 − V0) × 100, where V is the cell viability determined in each well with or without prior sample treatment, V0 is the averaged viability of cells exposed to ethanol without sample treatment. Assays for isolation of active compounds were repeated twice. All other assays were repeated at least three times. In all cases, the assay results were reproducible.
Statistical analysis Data values were expressed as mean ± SD. Data were analyzed by Dunnett's one-sided test or Tukey-Kramer's test. Statistically significant differences were indicated with * (P < 0.05) and ** (P < 0.01).
Hepatocytoprotective activity of the turmeric extract and curcuminoids The turmeric extract and curcuminoids did not show preventive effects against ethanol-induced injury in hepatoma cell lines such as HepG2 (data not shown). The established hepatoma cell lines do not express certain enzymes related to ethanol metabolism (Rueff et al., 1996). For example, normal HepG2 does not express CYP2E1, which is responsible for ethanol-induced oxidative stress, and unlike CYP2E1-expressing recombinant HepG2, it is not susceptible to ethanol-induced cell injury (Xu et al., 2005; Wu and Cederbaum, 2008). On the other hand, the primary cultured hepatocytes retain ethanol metabolizing enzyme activities, and are useful for assessing the cytotoxicity of ethanol (Donohue et al., 2005). Therefore, we used primary cells prepared from a rat immediately before each experiment. The wide variability in the cell's sensitivity to ethanol necessitated us to try various ethanol concentrations so that the viability after ethanol exposure would fall within a desirable range. Further, to minimize variability and to make the sample effects more discernible, we normalized the results to calculate the cytoprotective activity as described in the Materials and Methods section. The range of ethanol concentrations employed in this study may appear rather high compared to the concentrations (less than 0.5%) normally expected in blood after alcoholic beverage consumption. The concentration range was selected from our experience so that at least one concentration of ethanol within the range would result in about 50% reduction in viability of the primary cells and the cytoprotective activity of curcuminoids or turmeric extract would be measurable. Under our in vitro culture conditions, ethanol concentrations lower than the range used did not induce a sufficient level of injury in the primary cells for the cytoprotective activity assay.
As shown in Fig. 3, the cytoprotective activity of 50 µg/mL turmeric extract powder, which is equivalent to a curcuminoid concentration of 6 µM, was stronger than that of the 6 µM purified curcuminoid solution. Since this result suggested that some cytoprotective components other than curcuminoids are present in the turmeric extract, we screened for the active compounds from 10 g of the turmeric extract powder.
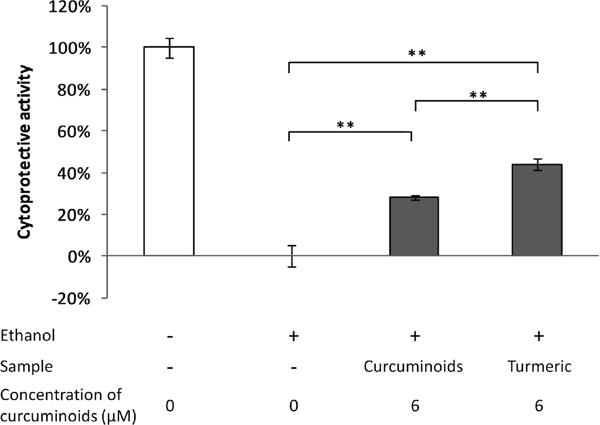
Cytoprotective activities of turmeric extract and curcuminoids against ethanol-induced hepatocyte injury. Hepatocyte cells were treated with 6 µM curcuminoids or 50 µg/mL turmeric extract (concentration of curcuminoids is 6 µM) for 16 h, and then exposed to 3.0% ethanol for 24h. Cell viability was determined by the resazurin assay. Cytoprotective activity against ethanol-induced hepatocyte injury is calculated as follows.
Cytoprotective activity (%) = (V − V0) / (100 − V0) × 100
where V is the cell viability determined in each well with or without prior sample treatment, V0 is the averaged viability of cells exposed to ethanol without sample treatment.
Values are expressed as mean ± SD (n = 4). Data are analyzed by Tukey-Kramer's test. Statistically significant differences were indicated with * (P < 0.05) and ** (P < 0.01).
Isolation of active compounds As shown in Fig. 1, the turmeric extract powder was first extracted with methanol, and the extract was separated into 10 fractions. Fractions 1 to 5 were more hydrophobic, and fractions 6 to 10 were more hydrophilic than the curcuminoids. Based on the assay results of hydrophobic fractions, active fraction 2 was further fractionated to obtain fractions 2-1 to 2-5, and compound 1 was finally isolated from active fraction 2-2 with a calculated yield of 3.2 mg/10 g turmeric extract. Based on the assay results of hydrophilic fractions, compound 2 was isolated from active fraction 7 with a calculated yield of 8.8 mg/10 g. Fraction 2-4 was also active, and two compounds were isolated from this fraction; however, the amounts isolated were so small that they were not used for further analysis.
Structural elucidation The structures of the isolated compounds were determined on the basis of mass spectrometry data and 1H- and 13C-NMR data. Comparison of these NMR data with those in the literature revealed that compounds 1 and 2 were ar-turmerone and bisacurone, respectively (Fig. 4) (Sasaki et al., 2003; Uehara et al., 1989). The MS data of compounds 1 and 2 were in agreement with the calculated mass of ar-turmerone and bisacurone.

Structures of compounds 1 and 2 isolated from active fractions.
Comparison of activity between curcumin and isolated compounds Cytoprotective activity of ar-turmerone and bisacurone, both of which were isolated in this study, were compared with that of curcumin (reagent grade) at concentrations of 1 µM and 6 µM. While all three compounds showed comparable preventive effects against ethanol-induced injury at 6 µM, only bisacurone was effective at concentrations as low as 1 µM (Fig. 5). Since the cytoprotective activities calculated for all samples were positive, the presence of cytotoxicity was considered unlikely. If a test sample had exerted cytotoxicity, the viability of cells treated with the test sample and exposed to ethanol would have been lower than that of the cells exposed to ethanol without sample treatment, and yielded a negative value in the calculation of cytoprotective activity.

Cytoprotective activities of reagent curcumin, isolated ar-turmerone, and bisacurone against ethanol-induced hepatocyte injury. Cells were treated with curcumin reagent, purified ar-turmerone, or purified bisacurone at two concentration (1 µM and 6 µM) for 16 h, and then exposed to 1.5% ethanol for 24h. Cell viability was determined by the resazurin assay. Cytoprotective activity against ethanol-induced hepatocyte injury is calculated as in Fig. 3. Values are expressed as mean SD (n = 5). Data are analyzed by Dunnett test with the alternate hypothesis; µsample > µcontrol. Statistically significant differences from no test-sample controls were indicated with * (P < 0.05) and ** (P < 0.01.)
Concluding remarks In the present study, we isolated ar-turmerone and bisacurone from the active fractions of a turmeric extract powder. These compounds are bisabolane-type sesquiterpenes. ar-Turmerone is the major component of turmeric essential oil and has been reported to possess many biological activities, as observed with curcuminoids. Bisacurone, a rare compound occurring in minute amounts in turmeric, was isolated from Curcuma xanthorrhiza in 1989 for the first time (Uehara et al., 1989). Its anti-inflammatory activity was reported, but other biological activities have been hardly demonstrated (Chai et al., 2008). Industrial production of turmeric extract commonly employs organic solvents to achieve a high yield of curcuminoids and essential oil constituents. Since the turmeric extract used in this study was obtained by hot water extraction, the extract could have contained more hydrophilic bisacurone at higher concentrations. Bisacurone showed a preventive effect against ethanol-induced hepatocyte injury at a lower concentration than curcumin or ar-turmerone. It was suggested that there were more active compounds in turmeric in addition to curcuminoids, ar-turmerone, and bisacurone, which contributed to the preventive effect against ethanol-induced hepatocyte injury. To assess the contribution of each compound to the net cytoprotective activity of turmeric extract, reconstitution experiments will be required.
The fact that the turmeric extract showed preventive effects against ethanol-induced injury in primary cells, but not in hepatoma cell lines such as HepG2, suggested that the cytoprotective mechanism of these compounds could involve functions retained by the primary cells, but not by the hepatoma cell lines. We are currently investigating the mechanism using DNA microarray analysis.
It is known that alcohol hangover is related to a deterioration of job performance, and an increased health risk (Wiese et al., 2000). The cure for hangover therefore is of societal concern. Hangover symptoms have been attributed to several causes, including the direct effects of alcohol, and the effects of compounds produced as a result of alcohol metabolism, especially acetaldehyde (Swift and Davidson, 1998). Therefore, the maintenance of liver function should play a role in recovery from hangover.
In this paper, we showed that pretreatment of hepatocytes with turmeric extract before exposure to ethanol had a preventive effect against cell injury, and in addition to curcuminoids, we isolated ar-turmerone and bisacurone as active compounds from the fractions of turmeric extract powder. Our results suggest that it is not a single component but a variety of components in turmeric that are involved in the prevention of alcohol hangover. As both hydrophobic and hydrophilic components showed preventive activity, extraction methods likely play an important role in the preparation of effective turmeric extracts.
Acknowledgements We thank Dr. Teruo Kawada of Kyoto University and Dr. Taiichiro Seki of Nihon University for technical help with the isolation of hepatocytes. We also thank Dr. Hisanori Kato of Tokyo University for the useful advice and suggestions.