2017 年 23 巻 2 号 p. 355-361
2017 年 23 巻 2 号 p. 355-361
There are few reports on the combined effect of epigallocatechin gallate (EGCG) and quercetin (QU) on the arteriosclerosis index (AI) in mice fed a normal diet. The effects of diets with high flavonoid contents on body and liver weights, and AI were investigated in C57BL/6 J mice. Five-week-old C57BL/6 J mice were fed diets containing EGCG (0.01 – 1.0%), QU (0.01 – 1.0%), or EGCG + QU (0.03% each) for 4 weeks. After breeding, body and liver weights were measured. Blood samples were collected by cardiac puncture and total, HDL, and LDL cholesterol levels were measured. The flavonoid containing diets appeared to have slight effects on both body and liver weights. Cholesterol levels changed slightly after breeding of mice fed any of the three diets. The results indicated that these flavonoids have very slight effects on weight gain and liver weight. AI was significantly reduced in breeding mice fed the combination (EGCG + QU) diet. These results suggest that the combination of EGCG and QU is beneficial for the maintenance of cardiovascular health.
Life-style related diseases such as hypertension, hyperlipidemia, and obesity are a social problem, and it is necessary to mitigate their symptoms on human health, quality of life, and longevity. Food and supplement therapies are suggested to reduce cholesterol levels in blood, which may be effective in controlling hyperlipidemia. Hyperlipidemia is a disease characterized by an increase in total cholesterol level or low-density lipoprotein (LDL) cholesterol level. This condition increases the risk of cardiac or cerebral infarction. Therefore, it is recommended that these patients reduce their serum levels of LDL cholesterol.
Recently, a new health claim system for human health was developed, and many health foods or supplements are now being shipped to market. We expect the serum cholesterol reducing effect of these foods and supplements will decrease the risk of some infarctions.
Non-nutrient components of foods are thought to have beneficial effects on health. Polyphenols are an important component of foods, specifically the flavonoids and carotenoids found in vegetables, fruits, and cereals. There are many types of polyphenols including glycosides, and it is well known that these components have various beneficial effects on lifestyle-related diseases. For example, tea catechins are well-known flavonoids. There are a number of reports that these tea catechins have antihypertensive (Negishi et al., 2004; Li et al., 2013a; Li et al., 2013b) and antihyperlipidemic effects (Ahmad et al., 2015; Yousaf et al., 2014). The antihyperlipidemic effects of quercetin (QU) in a high-fat diet-induced obesity model have also been reported (Jung et al., 2013).
In these reports, experiments using high-fat and high-sugar diet models were performed. While a few reports employed normal breeding conditions, it was difficult to detect differences between groups fed the flavonoid- or normal-diet. Furthermore, there are few reports on the intake of large amounts of flavonoids in animals. It is important to investigate the effects of high flavonoid intake, because data on the health benefits of supplementary flavonoid intake is required for the marketing of health foods.
Some reports have also shown that combinations of flavonoids showed enhanced anti-inflammatory activities (Funaro et al., 2016), while a combination of flavonoid and telomerase inhibitor (originating from epigallocatechin gallate (EGCG)) enhanced cancer cell proliferation (Chung et al., 2016).
Therefore, we investigated the effects of diets high in EGCG and QU on cholesterol levels in mice. The arteriosclerosis index (AI) was also used to measure the effects of EGCG and QU, independently and in combination.
Agents EGCG was purchased from Toronto Research Chemicals, Inc. (Toronto, ON, Canada). QU dehydrate was purchased from LKT Laboratories, Inc. (St. Paul, MN, USA).
Laboratory Animals C57BL/6 J mice were purchased from Charles River Laboratories Japan, Inc. (Yokohama, Japan). The animals were fed a standard powder diet (NMF, Oriental Yeast Co., Ltd., Tokyo, Japan) and tap water ad libitum with a 12-hour light/dark photoperiod during acclimatization. The experiment was conducted according to the ethical norms approved by the Animal Ethics Committee Guidelines of the National Food Research Institute, NARO.
Experimental procedure The powder diet was mixed with EGCG or QU at the concentrations of 0.01, 0.03, 0.1, 0.3 and 1.0% (w/w) individually, and at 0.03% each in combination. Five-week-old C57BL/6 J mice were fed the diets using a Roden Cafe® (a food case with cover) ad libitum for 4 weeks under temperature and humidity conditions of 24 ± 3°C and 40 – 60%, respectively. The body weights of mice were measured over several days. After breeding, blood samples were collected from mice by cardiac puncture. The animals were then euthanized by cervical dislocation, and liver weights were measured. The EGCG, QU, and EGCG + QU diet experiments were carried out independently.
Serum cholesterol assay Total cholesterol, high-density lipoprotein (HDL) cholesterol, and LDL cholesterol levels were measured using an EnzyChrom™ AF HDL and LDL/VLDL Assay Kit (BioAssay Systems, Hayward, CA, USA).
Statistical Analysis All data were expressed as the mean ± SEM. Data were evaluated by one-way analysis of variance (ANOVA) before Dunnett's multiple comparison test. Differences were considered significant at p<0.05.
The effect of an EGCG-containing diet on body-weight gain during the feeding period was measured. Figure 1A shows the weight gain of mice fed the 0.01, 0.03, 0.1, 0.3, and 1.0% EGCG-containing diets. The EGCG-added diets reduced body-weight gain in a dose-dependent manner (Fig. 1B). However, the reducing effect of the 1.0% EGCG diet was not significantly different from that of the control group (p = 0.2166). The effect of EGCG on normal body-weight gain in mouse experiments (Jang et al., 2013) and human trials (Chen et al., 2016) was previously reported. Our results were similar to those reported in the preceding papers. We also simultaneously measured diet intake during the feeding period. However, there were no differences between the EGCG- and control-diet groups (data not shown). These results suggest that EGCG has a small reducing effect on normal weight gain. Specifically, the reducing effect represented a 5.5% decrease as compared to the normal diet group.

Changes in body weight during the feeding period and final body weight after 28 days of feeding. C57BL/6 J mice were fed EGCG (A and B), QU (C and D), or EGCG + QU (E and F)-containing powder diet for 4 weeks. Contents of EGCG and QU powder diets were from 0.01% to 1.0%. The EGCG + QU diet contained 0.03% of each component.
The effect of a QU diet on body-weight gain during the feeding period was also measured. Figure 1C shows the weight gain of mice fed the 0.01, 0.03, 0.1, 0.3, and 1.0% QU-supplemented diet. QU did not show any toxicity or side-effects in this experiment. There was a dose-dependent slight increase in body-weight gain (Fig. 1D); however, the increasing effect of QU was small, ca. 5.0%. This appears to be a very slight effect. The measurements from the highest-dose group (1.0%) were not significantly different from those of the control group (p = 0.3069). It has previously been reported that QU has a reducing effect on body-weight gain (Hoek et al., 2014; Hoek et al 2015). In contrast, it was also reported that QU increased body weight in rats (Selvakumar et al., 2012). In the preceding study, Wistar rats weighing 180 – 200 g (90 days) were used. It is noteworthy that our experimental conditions were very different from the preceding rat study conditions. However, it would be of interest to further investigate the action of QU on body-weight gain.
In the combination (EGCG + QU) group, a 0.03%-diet was selected as a reasonable supplemented diet from calculations of the diet intake in mice. Combination-effect experiments were performed with the 0.03%-diet under similar conditions. Figure 1E shows the weight gain of mice over a 4-week period. There were almost no differences among the groups during the study period. The study diet showed no toxicity or side-effects, and there were no differences in dietary intake among the groups (data not shown). At the end of the study period, the final body weights were not significantly different between the groups. The body weights of the combination group increased slightly compared to the control, although it is not significantly increase (Fig. 1F). The combination effect of EGCG and QU on body-weight gain is notable, and more detailed research should be performed in future to further investigate the relationship. The combination effects of EGCG and QU were previously studied as an anticancer therapy (Wang et al., 2012a; Wang et al., 2012b; Wang et al., 2014; Hsieh et al., 2009). Wang et al. reported that there were no differences in the body weights of SCID mice (treated with a combination of EGCG and QU) (Wang et al., 2012b). More precise experiments are needed to confirm this observation.
To study the effects of EGCG, QU, and EGCG + QU, the liver weight in healthy animals was measured before and after breeding. The liver weight was expressed in relative terms, as a percentage of the body weight (Fig. 2). There were almost no significant changes in EGCG-treated C57BL/6 J mice (Fig. 2A). In the QU experiments, there was a significant increase in the liver weight of mice at the highest dose (1.0%) diet (Fig. 2B). These results indicate that EGCG is a safe food component and that QU is relatively important for liver homeostasis. However, from calculations using food intake values, a 1.0% diet translates to approximately 1.0 g/kg bodyweight/day. It is clearly not possible to consume this amount in a normal diet. Recently, the hepatoprotective effects of QU were reported. (Chiş et al., 2016; Hädrich et al., 2016). The responsible mechanism has been suggested to involve antioxidative and anti-inflammatory effects. It seems that this protective effect of QU is involved in the observed increase in liver weight. To clarify this mechanism of QU, more precise experiments are needed. Figure 2C shows the results for liver weight in EGCG- (0.03%), QU- (0.03%) and EGCG + QU-diet experiments. The relative liver weights of EGCG, QU, and the combination diet groups showed a tendency to decrease compared to the control diet group; however, these decreases were no significant. In Figure 2, the control values (content 0) showed some variability. The fluctuations in the control values might be attributed to the strain of mice used and the breeding conditions. Therefore, more precise experiments are needed to clarify this point.
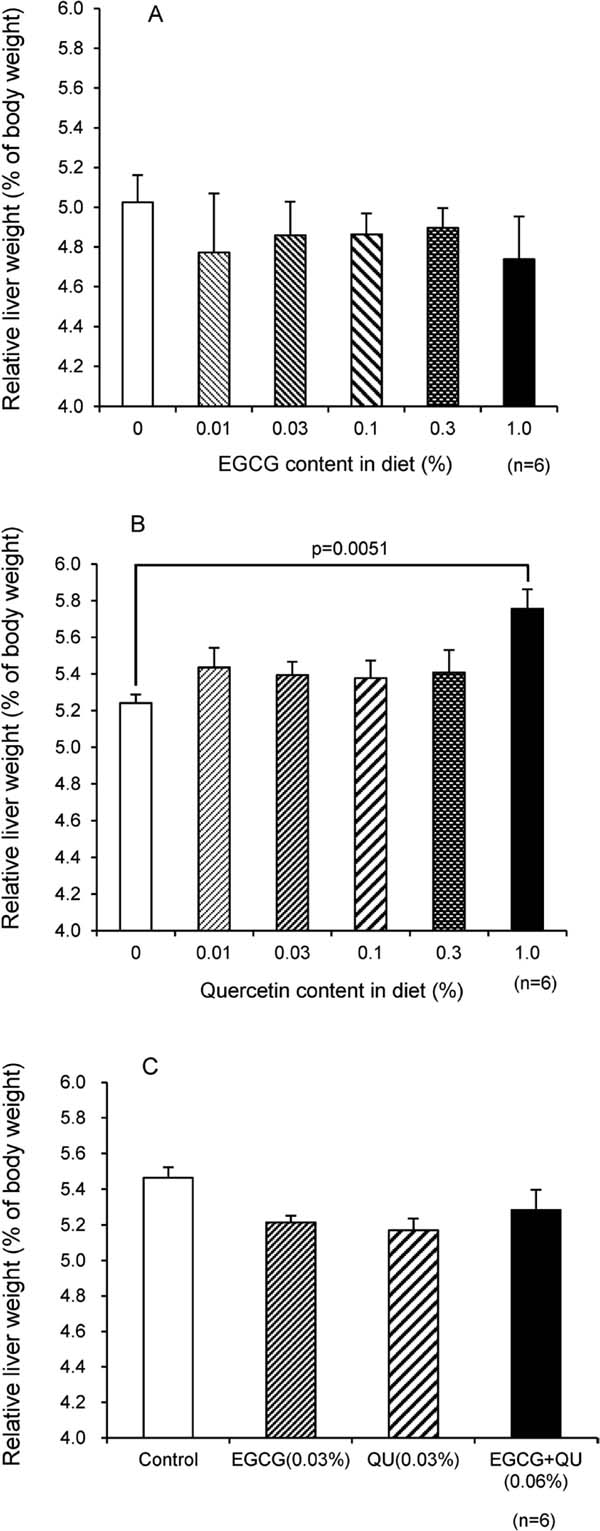
Relative liver weights of C57BL/6 J mice at the end of the feeding period. Graphs show the EGCG (0.03%) (A), quercetin (0.03%) (B), and EGCG + QU (0.03% each) diets (C). After breeding with the flavonoid diets, the mice were sacrificed by cardiac puncture and cervical dislocation. The livers were removed and weighed. The liver weights were calculated relative to the body weight. The values are expressed as mean ± SEM. The ANOVA value of Fig. 2B was p=0.030. “p” in Fig. 2B indicates the probability of Dunnett's test.
Cholesterol levels in serum and the AI were investigated after feeding EGCG, QU, and their combination to mice. There was no change in the total cholesterol level of the EGCG-diet group (Fig. 3A). Furthermore, AIs were obtained from a calculation of HDL- and LDL-cholesterol values (Fig. 3B). The index gradually increased in a dose-dependent manner. However, the increase in AI was not significantly different compared to the control group. Also, the QU diet did not appear to influence total cholesterol level in serum at the end of the feeding period (Fig. 3C). Although the changes in AI were not significant, the AI in groups fed the 0.01% to 0.1% QU diet was gradually reduced, while that in groups fed the 0.3% to 1.0% QU diet increased (Fig. 3D). Figure 3E shows the total cholesterol level in the serum of mice. Cholesterol levels in the EGCG, QU alone diet groups did not differ significantly from the control group. Also, the total cholesterol level of the combination group did not differ significantly from the control group. The EGCG group showed a small decrease, while the QU group showed a somewhat larger decrease in AI. However, these changes were not significantly different from the control group. Meanwhile, in the EGCG + QU group, AI was significantly reduced compared to the control group (p=0.0185, Fig. 3F), indicating that the combination diet had the greatest reducing effect on AI. This result is a novel finding on the effects of flavonoids in regards to their food function. However, the mechanism of action remains to be clarified. Notably, the combination effect was determined to be additive, and not synergistic. This suggests that each flavonoid acts at a different site. For example, low concentrations of EGCG were able to suppress intracellular lipid accumulation (Furuyashiki et al., 2004), suggesting that the cholesterol reducing effects of EGCG are due to its influence on intestinal lipid absorption. QU has a number of antioxidant, anti-inflammatory (Kim & Park 2016) and antiplatelet effects (Landolfi et al., 1984). It seems likely that the sites of action of these flavonoids are different. Synergistic effects were reported for curcumin and EGCG (Eom et al., 2015). These compounds exhibited antiproliferative activity against a cancer cell line; however, different sites of action were involved. In our experiments, it was challenging to identify a clear effect because the action of the flavonoid alone was weak. To clearly identify the action of the flavonoid, we need to improve the precision of the experiments.

Total cholesterol levels in the serum of mice, and arteriosclerosis index (AI). Graphs show the total cholesterol level with breeding of EGCG (A), quercetin (C), and EGCG + QU (E) diets. AI of mice fed EGCG (B), quercetin (D) and EGCG + QU (0.03% each) (F) diets. Total cholesterol level in serum was measured using an assay kit, and AI was calculated from LDL- and total-cholesterol levels. The values are expressed as mean ± SEM. The ANOVA value of Fig. 3F was p=0.032. “p” in the Fig. 3F indicates the probability of Dunnett's test.
Several health functions of flavonoids have been reported. For example, the preventative effects on lifestyle-related diseases including antihypertension, antihyperlipidemia, and liver function improvement were recently reported (Li et al., 2013a; Li et al., 2013b; Hashem et al., 2016; Bao et al., 2016; Liang et al., 2016). Researchers reported the synergistic anti-inflammatory effects of a combination of two flavonoids (Funaro et al., 2016). Meanwhile, others reported a combination effect of antibiotic drugs and flavonoids (Navrátilová et al., 2016). However, there are few reports dealing with the combination effects of two flavonoids. To our knowledge, this is the first report on the effects of a two-flavonoid combination diet. In the consumption of various foods, such as vegetables, fruits, cereals etc., humans are exposed to more than one flavonoid. The intake of multiple flavonoids, specifically EGCG and QU, could contribute to a healthy lifestyle, and reduce the risk of life-style related diseases.
Acknowledgments This work was partly supported by funds for the Research Project on Development of Agricultural Products and Foods with Health-promoting Benefits (National Agricultural and Food Research Organization).