2017 Volume 23 Issue 3 Pages 395-402
2017 Volume 23 Issue 3 Pages 395-402
Roasted wheat bran flour (RWBF) was fermented by Bacillus subtilis HA to produce bioactive compounds including mucilage and peptides. A moisture content of 60% was optimal for the solid-state fermentation, which exhibited the highest mucilage productivity after 1 day. Adding MSG to the substrate resulted in increased viable cell counts and mucilage and decreased acidity. After 1 day of fermentation, the RWBF containing 5% MSG indicated pH 6.5, mucilage content of 11.5%, consistency index of 5.59 Pa·sn, viable cell counts of 6.25 × 108 CFU/g, 4.0 mg/g tyrosine equivalents, and protease activity of 20 U/g. The total content of free amino acids tended to decrease during the alkaline fermentation, in particular, that of glutamate, indicating that the added MSG was efficiently converted to poly-γ-glutamic acid, with an 80% yield. The proposed solid-state fermentation is an effective approach to produce a highly valuable ingredient from wheat bran.
Wheat is the third most produced grain crop in the world after corn and rice (i) and is an important staple for many people around the world. The wheat kernel is composed of approximately 83% endosperm, 14.5% bran, and 2.5% germ. Wheat milling, in which the endosperm is separated to make white flour, generates bran and germ as by-products (ii); these by-products contain healthy compounds and could be incorporated into foods to improve their nutritional and functional properties. Wheat bran is rich in vitamins B and E, minerals and dietary fiber, which is crucial for gut health and also exhibits cardiovascular, antiobesity, and anticancer benefits. In addition, wheat bran exhibits antioxidant activity (Stevenson et al., 2012). However, apart from all-bran cereals, wheat bran is commonly used in animal feeds instead of foods because of its tendency to spoil quickly because of its content of fatty acids and enzymes (iii). Thus, it is necessary to develop processing technologies that would allow incorporating wheat bran or its components to foods to enhance their health attributes, while maintaining acceptable sensory properties. One approach could be fermentation, which can enrich the bran substrate with bioactive compounds and at the same time decrease the content of phytate, an antinutrient (Stevenson et al., 2012).
Bacillus subtilis is a Gram-positive bacterium that is used to make Oriental fermented foods such as natto and chungkukjang (Kim et al., 2014). Fermentation with B. subtilis can produce valuable biological compounds, in particular mucilage composed of poly-γ-glutamic acid (γ-PGA) and levan, which can provide functional and organoleptic benefits when incorporated to foods (Shih and Yu, 2005; Kim et al., 2014). γ-PGA is a highly viscous polypeptide composed of glutamate units; it is nontoxic, biodegradable and has high water absorption capability. γ-PGA applications include food thickener, humectant in cosmetics, drug carrier, and water treatment among others (Jian et al., 2005). Moreover, γ-PGA exhibits health benefits including anticancer effects, immune stimulation, and enhanced calcium absorption (Kim et al., 2014). Levan is a fructose polymer produced from sucrose-based substrates; it can be used as thickener and prebiotic in food and also as plasma extender, drug carrier, and adhesive. In addition, levan exhibits antioxidant, antiinflammatory, and cholesterol-lowering effects (Srikanth et al., 2015).
Therefore, the objective of this study was to fortify wheat bran with bioactive compounds such as mucilage and peptides, aiming to produce a novel functional food ingredient based on this abundant agro-industrial by-product.
Materials Roasted common wheat bran flour (RWBF, from Triticum aestivum subsp. aestivum) was obtained from Yoosmile Co. (Kyungpook, Korea) and kept at −20°C. Monosodium glutamate (MSG) was purchased from Yakuri Pure Chemicals Co. Ltd. (Kyoto, Japan).
Analytical assays General compositional analysis of the RWBF was performed according to the Korean Food Codex (iv). The moisture content was determined by weight loss after drying at 105°C. Crude protein was estimated using the Kjeldahl method (apparatus Buchi 339, Buchi K-435, Flawil, Switzerland); briefly, the sample was digested and the released nitrogen (N) was used to estimate the amount of protein, applying the equation: N × 6.25. Crude fat and crude fiber were determined using the Soxhlet and Henneberg-Stohmann method, respectively. Inorganic compounds were determined by inductively coupled plasma (ICP) emission spectroscopy analysis.
To determine the particle size of the RWBF, a sample was suspended in 70% ethanol (1%, w/w) and subjected to a zeta potential and particle size analyzer (ELSZ-2000, Otsuka, Japan).
Starter preparation Bacillus subtilis HA (KCCM 10775P) was isolated from traditional fermented soybean and deposited in the Korean Culture Collection Center (KCCM). A 5% (w/v) defatted soybean suspension was prepared by homogenizing at 10,000 rpm for 1 min, followed by autoclaving at 121°C for 15 min. The inoculum was grown on an MRS agar plate for 24 h at 42°C, then transferred to the starter culture broth (50 mL) and incubated by shaking at 180 rpm and 42°C for 24 h.
Solid-state fermentation The RWBF was mixed with water and fortified with various MSG concentrations (0% – 5%), followed by autoclaving at 121°C for 15 min. The starter culture was inoculated at a 1% concentration and incubated (MIR-553, Sanyo Electric Biomedical Co. Ltd., Osaka, Japan) at 42°C for 3 days.
pH, acidity,and viable cell counts The pH of the fermented RWBF was determined by pH-meter (Model 420A, Thermo Orion, Beverly, MA, USA). Titratable acidity (%, w/v) was determined as lactic acid by measuring the amount of 0.1 N NaOH required to reach a pH of 8.3. Viable cell counts were determined as colony forming units (CFUs)/g by serial dilution followed by culturing on an MRS agar plate.
Mucilage content The fermented RWBF (5 g) was mixed with 20 mL of water and centrifuged at 8,000 rpm for 20 min. The supernatant was mixed with twice the volume of isopropanol, and the precipitate was washed with 95% ethanol and dried at 50°C for 24 h to yield crude mucilage.
Consistency analysis Crude mucilage was dissolved in a 50-mM buffer solution (sodium phosphate pH 6.5, sodium acetate pH 4.5) and its consistency was determined using a rheometer (HAKKE RheoStress 1, Karlsruhe, Germany) fitted with a cone plate device (Plate PP35Ti, diameter 3.5 cm). A diluted mucilage sample was loaded onto the plate, and the shear stress (Pa) was measured against the shear rate (1 – 100 1/s) at 20°C. The consistency index was calculated by the Power law model (Genç et al., 2002), as follows:
 |
Where σ = shear stress (Pa), K = consistency index (Pa·sn), 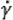 = shear rate (1/s), n = flow behavior index.
= shear rate (1/s), n = flow behavior index.
Protein hydrolysis To determine the proteolytic activity, a modified Anson's method was applied (Oh et al., 2006). Briefly, the fermented RWBF (4 g) was diluted 10-fold using phosphate buffer (20 mM, pH 7.0) and centrifuged at 15,000 rpm for 15 min. The supernatant was combined with 0.35 mL of a 0.6% casein solution and incubated at 37°C for 30 min, then mixed with the same volume of 0.44 M trichloroacetic acid (TCA) to stop the reaction and centrifuged at 15,000 rpm for 10 min. Subsequently, 1 mL of the supernatant obtained was reacted with 2.5 mL of 0.55 M Na2CO3 and 0.5 mL of Folin-phenol reagent. After incubating at 37°C for 30 min, absorbance was measured using a spectrophotometer (Ultrospec®2100 pro, Amersham Biosciences, Piscataway, NJ, USA) at 660 nm.
One unit of proteolytic activity was defined as the amount of protease releasing 1 g tyrosine equivalent/min. The tyrosine equivalents, including tyrosine, tryptophan, phenylalanine, and peptides released by protein hydrolysis, were estimated from a standard curve.
Analysis of free amino acids The fermented RWBF was extracted with distilled water and dried. The sample (0.5 mg) was reacted with 20 µL of a phenylisothiocyanate (PITC) solution (methanol:H2O:TEA:PITC=7:1:1:1) at room temperature for 30 min. After drying, the sample was dissolved in 200 µL of solvent A (140 mM sodium hydrogen acetate, 0.1% triethanolamine, 6% acetonitril; pH 6.1) and filtered through a 0.45-µm syringe filter. To analyze the total amino acid content of crude mucilage, dried mucilage was dissolved in 6 N HCl buffer, followed by heating and drying under vacuum to prepare the derivatives of amino acids.
Free amino acids were analyzed using a Hewlett Packard 1100 Series HPLC (Palo Alto, CA, USA) fitted with a C18 column (Waters Nova-Pak 4 µm, Milford, MA, USA). The mobile phase was eluted in gradient mode, starting with 100% Solvent A and ending with 100% solvent B (60% acetonitrile), during 25 min. The flow rate was 1 mL/min, and UV absorbance was determined at 254 nm.
Statistical analysis Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS Version 20.0, SPSS Inc. Chicago, IL, USA). The mean and standard error were calculated. Significant differences among means were determined by one way-ANOVA and Duncan's multiple range test (p < 0.05).
Physicochemical analysis of the RWBF The proximate composition and mineral content of the RWBF is summarized in Table 1. Carbohydrate was the major component (71.71%), followed by protein (15.74%) and fiber (7.58%). The RWBF was low in moisture (2.88%). The most abundant minerals in the RWBF were potassium (10.22 mg/g), phosphorous (9.43 mg/g), and magnesium (4.37 mg/g). Wheat bran is thus a valuable by-product of milling, rich in nutrients and dietary fiber, but which has a short shelf life because of the presence of lipids and lipolytic enzymes (iii). Thus, the wheat bran flour was sterilized to inactivate enzymes, eliminate indigenous microorganisms, and ensure the preservation of its organoleptic properties, making it a suitable material for fermentation.
| Component | Content (%) | |
|---|---|---|
| Moisture | 2.88 | |
| Crude protein | 15.74 | |
| Carbohydrate | 71.71 | |
| Crude fiber | 7.58 | |
| Crude fat | 5.35 | |
| Minerals | Mg | 4.37 mg/g |
| Ca | 0.83 mg/g | |
| K | 10.22 mg/g | |
| Mn | 0.14 mg/g | |
| P | 9.43 mg/g |
In addition to its dietary benefits, wheat and rice bran exhibit a high water absorption ability, of 3.54 and 2.47 g water/g bran, respectively (unpublished results). High water absorption capability is a crucial factor for substrates used in solid-state fermentation as a high water activity is required to ensure the microbial growth and occurrence of biochemical reactions. Substrates such as coconut husk and apple pomace exhibit high water absorption capacity, allowing adequate microbial growth during solid-state fermentation (Orzua et al., 2009). Hence, apart from being a rich source of nutrients and fiber, the high water absorption capability of the RWBF can provide suitable culture conditions for the solid state fermentation with Bacillus subtilis HA.
Effect of initial moisture on the solid-state fermentation with B. subtilis HA The initial moisture content of the substrate is critical for the success of solid-state fermentation because microbial growth and generation of products occurs at the interface between the moist substrate and the environment (Mukherjee et al., 2009). Therefore, optimization of the initial moisture content, which determines the water activity of the substrate, is of paramount importance for an effective solid-state fermentation.
Here, 30 g of RWBF was mixed with water at ratios of 1:1 – 1:3 (w/w; equivalent to moisture contents of 50% – 75%), followed by the addition of B. subtilis HA starter at a 1% concentration (w/w), and the mixture was fermented at 42°C for 24 h.
The effect of the moisture content on the mucilage production, viable cells, tyrosine equivalents, and protease activity is summarized in Table 2. The fermented RWBF with 50% moisture showed low tyrosine equivalents and non-detectable mucilage, although it had a high viable cell count and the highest protease activity. A moisture content of 60% resulted in the highest amount of mucilage produced (8.5%), without adding MSG, as well as the highest tyrosine equivalents (4.73 mg/g) among the treatments. Mucilage production, tyrosine content, and protease activity decreased as the moisture content increased, even though the number of viable cells showed the opposite tendency. Although the optimal moisture content varies according to the microorganism, the result obtained agrees with previous reports describing the detrimental effect of excess moisture on solid-state fermentation (Baysal et al., 2003; Jian et al., 2005), resulting in lowered substrate porosity and gas exchange (Mukherjee et al., 2009) and thus affecting the metabolic performance of B. subtilis, even though these facultative anaerobes are able to survive in poorly aerated conditions (Nakano and Zuber, 1998).
| Moisture content (%) | Mucilage content (%) | Viable cell counts (CFU/g) | Tyrosine equivalents (mg/g) | Protease activity (U/g) |
|---|---|---|---|---|
| 50 | 0.0±0.00d | 2.42×108 | 0.99±0.0e | 69±1.0a |
| 60 | 8.5±0.14a | 2.47×108 | 4.73±0.0a | 55±1.2b |
| 67 | 5.6±0.42b | 4.25×108 | 3.96±0.0b | 32±1.0c |
| 71 | 5.2±0.00b | 4.35×108 | 3.75±0.0c | 29.9±0.5d |
| 75 | 3.7±0.28c | 4.53×108 | 3.58±0.0d | 26.3±0.4d |
The high amount of mucilage obtained in optimal moisture conditions, even without adding MSG, indicates that RWBF is a suitable substrate for the solid-state fermentation with B. subtilis. The amount of mucilage obtained here (8.5%) was higher than that obtained by fermenting roasted soybean flour with a co-culture of B. subtilis and Lactobacillus plantarum (3.5%) (Park et al., 2012) and comparable to that attained by using B. subtilis KU-A and GT-D to ferment soy milk cake enriched with MSG (7.8% – 9.5%) (Oh et al., 2007), but was lower than that achieved by fermenting roasted soybean flour and mixed cereals with B. subtilis HA (10%) (Son and Lee, 2011).
Under optimal moisture conditions (60%) for mucilage production, the number of B. subtilis viable cells was 2.47×108 CFU/g, which increased as the moisture content increased. Nevertheless, as with the mucilage content, an increase in moisture resulted in a substantial decrease in tyrosine and protease activity, which exhibited the lowest values (3.58 mg/g and 26.3 U/g, respectively) when the moisture content was the highest (75%).
To evaluate other potential advantages of the RWBF substrate, the bulk density of the RWBF was compared to that of rice bran at 60% moisture content (data not shown). As a result, the RWBF exhibited higher bulk density, indicating higher porosity and consequently greater capacity for gas exchange for the fermentation with B. subtilis HA, whereas the soaked rice bran showed a compact appearance. This result suggests that, in addition to adequate moisture content, different substrates would result in different productivities for solid state fermentation, with wheat bran being a suitable choice for this process. Wheat bran has been successfully used as a substrate in solid state fermentation to produce amylases, fructosyl transferase, pectinase, and lactic acid (Couto and Sanromán, 2006).
pH, acidity, and viable cells To prepare the substrate for fermentation, 30 g of RWBF was mixed with water to reach 60% moisture content, and various concentrations of MSG (0% – 5%) were added to boost the production of mucilage (Shi et al., 2006; Park et al., 2012). Solid-state fermentation was performed at 42°C for 3 days. The changes in the pH and acidity during the fermentation are shown in Fig. 1a.

Changes in the (a) pH and acidity of the roasted wheat bran substrate during 3 days of solid-state fermentation by B. subtilis HA, (b) viable cells counts during the 3 days of fermentation, (n = 3)
An increase in MSG concentration resulted in an increase in pH and a decrease in acidity in the fermented RWBF. At 5% MSG concentration, the pH increased from 6.3 at the start of the fermentation to 8.5 after 3 days, whereas acidity decreased to almost zero. By contrast, the substrate lacking MSG exhibited a less marked increase in pH, from 6.4 to 7.5, and a final acidity of 0.3%.
It was observed that the pH increased gradually in all cases, whereas the change in acidity depended on the MSG concentration.
The substrates with 0% or 1% MSG showed an increase in acidity after 1 – 2 days of fermentation, reaching 0.75% in the substrate without MSG on Day 2, followed by a decrease in acidity by the end of the fermentation. By contrast, the acidity of the substrate with 3% MSG only increased on Day 1 (to 0.72%) and then decreased, and that of the substrate with 5% MSG decreased gradually throughout the fermentation.
The MSG concentration also affected the final number of viable cells of B. subtilis HA in the fermented RWBF (Fig. 1b). The B. subtilis HA inoculum was 2.95×107 CFU/g, and the number of viable cells increased after one day of fermentation, reaching 6.25×108 in the substrate containing 5% MSG. After a slight decrease in the CFU/g on Day 2 in substrates with 3% – 5% MSG, the number of viable cells markedly decreased after 3 days of fermentation, particularly in the substrate containing 3% MSG (2.53 × 107 CFU/g). The final number of viable cells in the substrate containing 5% MSG was 3.87×107 CFU/g. In all cases, a typical microbial growth curve was observed, in which the number of viable cells increases rapidly, stabilizes, and then decreases as nutrients in the substrate are depleted and metabolites accumulate.
Therefore, 1 day of solid-state fermentation of RWBF with B. subtilis HA can be considered to be adequate for producing mucilage and attaining a high number of viable cells.
Mucilage production The changes in the consistency index and mucilage content of the RWBF enriched with 0% – 5% MSG and fermented for 1 – 3 days are displayed in Fig. 2.

Changes in the mucilage content and consistency index of the roasted what bran substrate enriched with 0% – 5% MSG and solid-state fermented by B. subtilis HA for 3 days, (n = 3)
The RWBF without MSG showed the lowest mucilage content (8%), which decreased as the fermentation progressed. The mucilage content of the RWBF with 1% or 3% MSG was higher after 1 day of fermentation, reaching 9.8% and 10.8%, respectively, but also decreased afterward. By contrast, after 1 day of fermentation, the RWBF enriched with 5% MSG exhibited the highest mucilage content (11.5%), which remained constant until Day 2, and then decreased slightly to 10.6% after 3 days of fermentation.
The mixtures containing 1% or no MSG exhibited low consistency indexes of 0.20 and 0.05 Pa·sn, respectively, which remained constant throughout the fermentation; whereas those containing 3% – 5% MSG exhibited an increasing consistency index as the fermentation progressed. In particular, the RWBF containing 5% MSG exhibited the highest consistency index throughout the fermentation, which increased from 5.59 Pa·sn on Day 1 to 7.23 Pa·sn on Day 3 of the fermentation.
The increase in the mucilage content and consistency index was thus correlated with the increase in MSG concentration, which can be explained by the fact that B. subtilis HA is a glutamate-dependent mucilage-producing strain (Urushibata et al., 2002; Kim et al., 2014). Hence, a higher concentration of MSG results in greater production of mucilage, which in this case is likely to be a mixed biopolymer composed of γ-PGA, levan, and other polysaccharides (Shih and Yu, 2005; Kim et al., 2014). Nevertheless, in all cases the mucilage content decreased after 2 days of fermentation, which may be explained by the action of enzymes such as PGA depolymerases (Ashiuchi et al., 2003) and hydrolases (Suzuki and Tahara, 2003). Nevertheless, the steady increase in the consistency index observed in mixtures containing 3% or more MSG may be explained by the increase in pH resulting from alkaline fermentation (Fig. 1a) because γ-PGA changes into a linear random-coil conformation as the pH rises, resulting in increased viscosity (Park et al., 2012).
Generally, B. subtilis produces mucilage consisting of γ-PGA and levan, and the composition of the mucilage depends on the composition of the nutrient source (Shih and Yu, 2005). The mucilage produced by B. subtilis in a defined medium containing 3% MSG was composed of over 90% γ-PGA and other biopolymers, indicating a higher content of glutamic acid (Seo et al., 2008). By contrast, here, when using RWBF as a substrate, the content of glutamic acid in the crude mucilage was approximately 5%, suggesting that the mucilage could be composed of around 5% γ-PGA, with the major component being a levan biopolymer. However, when enriching the substrate with 5% MSG, the content of glutamic acid in the crude mucilage was almost 20%, suggesting higher γ-PGA content in the mucilage as a result of the conversion of glutamate into γ-PGA by B. subtilis during the solid-state fermentation (unpublished results).
Mucilage was produced in high amount even without the addition of MSG, suggesting that the sugars present in the RWBF were converted to levan and its fructooligosaccharides (Shida et al., 2002). As described previously, RWBF is rich in carbohydrates (>70%) with sucrose being the major component (Saunders and Walker, 1969), making it a suitable substrate for generating levan-rich mucilage. By fortifying the medium with glutamate, which is a precursor of γ-PGA (Urushibata et al., 2002), the mucilage production would increase as both levan and γ-PGA would be generated (Shih and Yu, 2005).
Protein hydrolysis The changes in the tyrosine equivalents and protease activity of the fermented RWBF are displayed in Fig. 3. The initial tyrosine equivalents in all samples were approximately 2 mg/g, and increased as the fermentation time increased. The tyrosine equivalents indirectly indicate the extent of protein hydrolysis (Lee and Lee, 2014), thus, the increase in released peptides indicates that protein was hydrolyzed throughout the fermentation. Protein hydrolysis in fermented foods is affected by a protease secreted by B. subtilis, which has optimum activity in alkaline conditions (Park et al., 2012). In addition to the increase in released peptides, protein hydrolysis also increases the pH as ammonia is released during protein breakdown (Terlabie et al., 2006). The RWBF without MSG exhibited the highest tyrosine equivalent content throughout the fermentation, indicating 4.18, 6.71, and 7.22 mg/g after 1, 2, and 3 days of fermentation. On the other hand, the RWBF with 5% MSG exhibited a less marked increase in the tyrosine equivalents, which indicated 4.00 mg/g after 1 day and reached 6.00 mg/g by the end of the fermentation.

Tyrosine equivalents and protease activity in the roasted wheat bran substrate enriched with 0% – 5% MSG and solid-state fermented by B. subtilis HA during 3 days, (n= 3)
By contrast, protease activity tended to increase after 1 day of fermentation and subsequently decreased. Because protease activity correlates with the tyrosine equivalents, the RWBF without MSG exhibited the highest protease activity (68 U/g) after 1 day of fermentation, which decreased afterward to 40 U/g by the end of the fermentation. On the other hand, the RWBF with 5% MSG exhibited the lowest protease activity throughout the fermentation, indicating the highest activity (20 U/g) after 1 day, which markedly decreased afterward to 5 U/g by the end of the fermentation. The lowered protease activity observed when increasing the MSG concentration may be explained by inhibition of the enzyme by excess glutamate (Moon and Parulekar, 1991; Ferrero et al., 1996).
Free amino acid content The changes in the free amino acid content of the RWBF enriched with 5% MSG and fermented for 3 days are summarized in Table 3. This MSG concentration was selected because it resulted in the greatest mucilage production and number of viable cells among samples. As mentioned previously, the tyrosine content tended to increase as the fermentation progressed. In addition, the content of most amino acids tended to increase as well. However, the total amino acid content decreased from 32.32 to 11.84 mg/g after 1 day of fermentation but subsequently increased, reaching 14.02 mg/g by the end of the fermentation. This result was mainly due to the marked decrease in glutamic acid, from 31.57 mg/g at the beginning of the fermentation to 6.19 mg/g by the end of the fermentation. This outcome suggests that glutamic acid was efficiently converted to γ-PGA (Jian et al., 2005; Oh et al., 2007).
| Amino acid | Days of fermentation | |||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Cys | 0.00 | 0.00 | 0.06±0.01 | 0.06±0.00 |
| Asp | 0.10±0.10 | 0.33±0.01 | 0.34±0.70 | 0.42±0.01 |
| Glu | 31.57±1.84 | 5.80±0.22 | 5.62±0.02 | 6.19±0.15 |
| Asn | 0.13±0.01 | 0.20±0.01 | 0.15±0.02 | 0.05±0.00 |
| Ser | 0.00 | 0.35±0.02 | 0.30±0.04 | 0.21±0.01 |
| Gln | 0.00 | 0.03±0.01 | 0.03±0.01 | 0.03±0.00 |
| Gly | 0.04±0.00 | 0.42±0.02 | 0.39±0.04 | 0.44±0.02 |
| His | 0.00 | 0.25±0.02 | 0.25±0.04 | 0.27±0.01 |
| Arg | 0.05±0.00 | 0.00 | 0.04±0.00 | 0.13±0.01 |
| Thr | 0.00 | 0.20±0.01 | 0.20±0.03 | 0.23±0.01 |
| Ala | 0.06±0.00 | 0.40±0.02 | 0.38±0.05 | 0.51±0.01 |
| GABA | 0.05±0.01 | 0.08±0.02 | 0.11±0.01 | 0.15±0.01 |
| Pro | 0.01±0.00 | 0.26±0.02 | 0.43±0.05 | 0.58±0.02 |
| Tyr | 0.00 | 0.49±0.03 | 0.59±0.08 | 0.67±0.01 |
| Val | 0.00 | 0.43±0.02 | 0.47±0.07 | 0.61±0.01 |
| Met | 0.00 | 0.19±0.01 | 0.20±0.03 | 0.20±0.00 |
| Cys2 | 0.00 | 0.00 | 0.04±0.01 | 0.04±0.01 |
| Ile | 0.00 | 0.36±0.02 | 0.36±0.05 | 0.43±0.01 |
| Leu | 0.00 | 0.49±0.03 | 0.55±0.08 | 0.70±0.01 |
| Phe | 0.16±0.01 | 0.58±0.03 | 0.70±0.05 | 0.77±0.01 |
| Trp | 0.12±0.01 | 0.71±0.06 | 0.84±0.09 | 0.94±0.05 |
| Lys | 0.03±0.00 | 0.28±0.02 | 0.34±0.03 | 0.43±0.01 |
| TOTAL | 32.34±1.88 | 11.84±0.56 | 12.39±1.53 | 14.02±0.33 |
In this study, B. subtilis HA was used for the solid-state fermentation of RWBF. The optimal conditions of fermentation for generating mucilage were 42°C, 60% moisture (1:1.5 w/w of bran : water), 5% MSG, and 1 day of fermentation. Supplementing the substrate with MSG resulted in increased mucilage production (11.5%) and viable cell counts (6.25×108 CFU/g) and decreased acidity (nearly 0%, pH 6.5). This process allows using an abundant by-product like wheat bran for producing a value added product rich in levan, γ-PGA, and peptides. The product generated could be used as a functional ingredient in foods or as a source of biopolymers for various applications.
Acknowledgements This work was supported by the Korean Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through the Technology Commercialization Support Program, funded by the Ministry of Agriculture, Food and Rural Affairs (No. 314082-3).