2017 Volume 23 Issue 4 Pages 495-502
2017 Volume 23 Issue 4 Pages 495-502
L-alanine is an important amino acid that constitutes proteins and provides biological advantages to the human body. This study examined conditions for the crystallization of L-alanine using liquid anti-solvents and ultrasound as an auxiliary tool. Effects of process variables on the distribution of particle size and external habit were investigated. Propanol was found to be the most appropriate anti-solvent. In addition, results of particle size analyzer and scanning electron microscope revealed that the particle size of L-alanine crystals increased with an increase in L-alanine concentration, injection rate, or crystallization temperature. Ultrasound also caused significant particle size reduction.
L-alanine is a non-essential amino acid and is also a major constituent in protein production. Additionally, L-alanine plays an important role in controlling energy conversion and storage for muscle tissues, the brain, and the central nervous system. It also plays important roles in the immune and metabolic systems of the human body (Gaikwad et al., 2010). Studies have revealed that L-alanine may accelerate liver metabolism, thus leading to a quick elimination of toxic wastes from the body (Newsholme et al., 2003). Furthermore, L-alanine is considered to be a good substitute for artificial sweeteners since it delivers high levels of sweetness in small doses. At present, L-alanine is used as a raw material for alitame, which is an artificial sweetener 2000 times sweeter than sugar (Leroy and De Vuyst 2004) (Leroy and De Vuyst 2004). In addition, L-alanine is currently used as a food additive to enhance enteral and parenteral nutrition, and as a flavor promoter in the food industry (Kumagai 2000).
Crystallization is a widely used separation and purification method for the production of fine crystal particles. Different techniques such as the use of anti-solvents, evaporation, and cooling are commonly employed to induce crystallization (Myerson 2002). It is worth noting that compared to evaporation or cooling techniques, anti-solvent processes using organic or inorganic liquids are simpler and require less energy. Since no thermal energy is involved in anti-solvent processes, they can be used without compromising thermally sensitive materials or the biological activity of drugs. Thus, anti-solvent processes have been mostly employed to crystallize pharmaceutical compounds for practical applications. Crystallization processes induced by the addition of an anti-solvent produce high supersaturation levels rapidly and therefore leads to extremely high nucleation rates. These high nucleation rates thus result in small-sized crystalline particles that contain increasingly amorphous domains (Kim et al., 2014).
In the anti-solvent crystallization process, choosing the proper liquids is critical. Process variables including crystallization temperature, type of anti-solvent, solution concentration, injection rate, and method for mixing anti-solvent and solution, have significant effects on particle size distribution, external habit, and the growth rate of crystals. In addition, ultrasound has been used as a supplementary tool by many researchers to manipulate the crystal properties. Relative studies (de Castro and Priego-Capote 2007; Li et al., 2006; Park and Yeo 2010) demonstrated the numerous advantages of ultrasound-assisted crystallization, including the induction of primary nucleation, inhibition of agglomeration, manipulation of crystal size distribution, and modification of polymorphism.
High-protein foods such as meat (chicken, beef, pork) and fish are good natural sources of L-alanine, which can be separated from these sources and purified using anti-solvent crystallization methods. However, since few literature reports exist on anti-solvent crystallization of L-alanine, we were prompted to study this technique with the assistance of ultrasound. Owing to the solubility of L-alanine in water, various organic solvents such as methanol, ethanol, and propanol were tested as anti-solvents. Process variables that may significantly influence the crystallization process, such as L-alanine concentration, injection method and rate, temperature, and ultrasound power and time, were investigated. Subsequently, the effects of these process variables on particle size distribution and external habit were evaluated using a particle size analyzer as well as a field emission scanning electron microscope.
Materials L-Alanine (CAS 56-41-7) was purchased from Sigma-Aldrich Chemical Company, Inc. (Milwaukee, WI, USA). Methanol, ethanol, and n-propanol were also purchased from Sigma-Aldrich and used as organic anti-solvents.
Apparatus and experimental process The device used for L-alanine crystallization is shown in Figure 1, and consists of a magnetic stirrer (Corning PC-420D, US), a glass beaker for crystallizing, a burette for dipping anti-solvent, and an ultrasound homogenizer (Microson XL-2000, US). The magnetic stirrer was used to agitate the L-alanine solution and the anti-solvent, and thereby accelerate the nucleation and growth of the crystals. Additionally, constant temperature was maintained and controlled by magnetic stirrer heater. The ultrasound homogenizer was designed for ultrasound wave generation with a constant frequency of 22.5 kHz and available power outputs from 5–15 W. The probe (3 mm OD × 75 mm L) was immersed in the center of the solution inside the beaker during the crystallization process.

Apparatus for crystallization of L-alanine using a liquid antisolvent: (A) glass beaker for crystallization, (B) solution injector, (C) magnetic stirrer, and (D) ultrasound generator.
For the crystallization experiment, a 10 mL sample containing L-alanine concentrations of 0.01 g/mL was placed in a glass beaker, to which was added 10 mL of anti-solvent. Afterwards, the mixed solvent system was agitated with a magnetic stirrer for 30 min at 25°C. Once crystallization had reached completion, the L-alanine crystals were filtered using a funnel and a filter paper. The filter paper was then placed in an oven and dried for 24 h at 60°C.
Effects of different process variables including anti-solvent type (methanol, ethanol, propanol), the injection speed (1.0, 10.0, 58.8 mL/min) and method (AIS = anti-solvent into solution, SIA = solution to anti-solvent) of anti-solvent, crystallization temperature (25, 35, 45°C), ultrasound power (5, 10, 15 W) and time (30, 60, 90 s) were investigated.
Crystal analysis The dried crystals of L-alanine were coated with platinum under an argon atmosphere, and the external shapes of the crystals were analyzed using a field emission scanning electron microscope (FE-SEM, Hitachi S-4300 & EDX-350). Average particle size and size distribution were examined by a particle size analyzer (PSA, Ankersmid CIS-50). The PSA determines the size of the crystal by measuring the time of transition for a laser beam to pass through each particle (Park and Yeo 2012).
Effect of anti-solvents types In the present study, due to the relatively high solubility of L-alanine in water (16.65 g/100 g of water, 25°C), water was used as a solvent (Malliga et al., 2012). Different organic solvents such as methanol, ethanol, and propanol were tested as anti-solvents for L-alanine crystallization. Experiments were employed by injecting 10 mL of anti-solvent into 10 mL of 0.01 g/mL L-alanine solution at 58.8 mL/ min and stirred for 30 min at 25°C. Crystallizations employing anti-solvents resulted in L-alanine crystals (Figure 2b–d) that appeared as white needles, while unprocessed samples produced crystals with an irregular table form (Figure 2a). Particle sizes of the crystals were also significantly reduced in the samples containing anti-solvents compared to those in the unprocessed samples. PSA results shown in Figure 3 indicate that the average particle size of L-alanine crystals significantly varied when different organic solvents were used as anti-solvent. For example, using methanol as an antisolvent resulted in the largest particle size of 39.2 µm, followed by ethanol (25.5 µm) and propanol (19.9 µm). Solvent properties such as dipole moment, dielectric constant, and the presence of hydrogen bonding interactions may also influence the mechanism for crystal growth. Park et al., (2006) noted that crystal size varied with the solubility parameter of the solvent, and that crystal size increased when the solubility difference between the solvent and anti-solvent decreased. In our study, L-alanine was dissolved in water, and methanol, ethanol, and propanol as anti-solvents were tested. The crystal size of L-alanine increased as the solubility parameter of the anti-solvent became closer to that of water, indicating crystal size obtained by methanol is the largest.

SEM photomicrographs of L-alanine crystals obtained using different organic solvents as anti-solvents: (a) unprocessed, (b) methanol, (c) ethanol, and (d) propanol.

Average particle size of L-alanine crystals using different anti-solvents, which were injected into L-alanine solutions (0.01 g/mL concentration) at a rate of 58.8 mL/min at 25°C.
Effect of different L-alanine concentrations Drug solution concentration is an important factor in the liquid crystallization process. The effects of different L-alanine concentrations (0.75, 1.25, and 1.50 g/mL) on particle size and external shape of L-alanine crystals were inspected. The anti-solvent methanol (10 mL) was injected into the L-alanine solution at an injection rate of 58.8 mL/min at 25°C. Figure 4(a)–(c) illustrates the particles crystallized from samples containing L-alanine concentrations of 0.75, 1.25, and 1.50 g/mL, respectively. As shown in Figure 4(a), L-alanine crystals obtained from 0.75 g/mL solutions were aciculate in form with a clear periphery, whereas higher L-alanine concentrations of 1.25 and 1.50 g/mL (Figure 4(b) and (c), respectively) afforded crystals that appeared bundled with irregular edges. The PSA results shown in Figure 5 confirmed that the average particle size of the L-alanine crystals decreased from 44.8 to 14.9 µm as the L-alanine concentration increased. Similar conclusions have been previously reported, with the interpretation that more highly concentrated solutions exhibit higher degrees of supersaturation upon mixing with the anti-solvent, which leads to higher nucleation rates (Kim et al., 2014; Park and Yeo 2012). Such high nucleation rates could be responsible for the formation of massive nuclei and increase the number of crystals, which result in smaller crystal sizes. In contrast, less concentrated solutions exhibit lower degrees of supersaturation and consequently produce fewer nuclei. This could lead to continuous crystal growth and thus, much larger crystals.

SEM photomicrographs of L-alanine crystals obtained using different L-alanine concentrations: (a) 0.75 g/mL, (b) 1.25 g/m, and (c) 1.5 g/mL at 25°C. An injection rate of anti-solvent (propanol) of 58.8 mL/min was used.

Average particle size of L-alanine crystals at different concentrations (0.75, 1.25, and 1.50 g/mL) of L-alanine solution. Propanol is injected into L-alanine solutions at an injection rate of 58.8 mL/min at 25°C.
Effect of different injection methods and rates Two injection methods commonly used in liquid crystallization include anti-solvent into solution (AIS) and solution into anti-solvent (SIA). Another factor to consider is the injection rate, which influences particle size during crystallization. In this study, experiments were performed using propanol as anti-solvent and L-alanine solutions at a concentration of 1.5 g/mL; the effects of the injection methods (AIS and SIA) and different injection rates (1.0, 10.0, 58.8 mL/min) on the crystal size of L-alanine were investigated. The crystallization temperature was controlled at 25°C. As shown in Figure 6, larger L-alanine particles were formed using AIS injection methods versus the SIA method. The PSA results in Figure 7 show the particle size distribution of L-alanine crystals using injection methods of AIS and SIA with injection rates of 1.0, 10.0, and 58.8 mL/min. Average particle sizes of L-alanine crystals varied from 21.3 µm to 47.9 µm when different injection methods and rates were used. For instance, faster injection rates of anti-solvent resulted in larger L-alanine crystals. Overall, the particle sizes of L-alanine crystals obtained through the SIA method are much smaller than those obtained by AIS regardless of injection rate. These results suggest that different injection methods and rates significantly affect the crystallization process, which is also dependent on the degree of supersaturation and mass transfer between the drug solution and anti-solvent (Matsumoto et al., 2013; Park and Yeo 2011). Rapid injection rates presumably accelerate mass transfer processes as well as supersaturation of target molecules, which results in higher nucleation rates. And Zhou et al. (2006) believed that the anti-solvent addition rate could increase the crystallization progresses due to the increase in the crystal surface area. As mentioned earlier, the increased nucleation rate could induce the formation of a substantial number of nuclei in the initial stage of nucleation process, and as a result, the crystal size will be diminished. In contrast, the initial nucleation rate decreases when mixing rate of drug solution and anti-solvent is slow, which would form much smaller number of nuclei, resulting in an increased crystal particle size.

SEM photomicrographs of L-alanine crystals obtained using different injection methods: (a) anti-solvent into solution (AIS), and (b) solution into anti-solvent (SIA). The L-alanine solution concentration is 0.01 g/mL with propanol as the anti-solvent.

Average particle size of L-alanine crystals at different injection rates. The L-alanine solution concentration is 0.01 g/mL, propanol is used as the anti-solvent employing either the solvent into anti-solvent (SIA) or anti-solvent into solvent (AIS)
Effect of different crystallization temperatures Temperature is another important factor that influences the crystallization process since heat transfer in the mixed solution and solubility of the targeted solute are closely associated with temperature changes. Accordingly, the effects of different temperatures (25, 35, 45, and 55°C) on L-alanine crystallization were investigated. Figure 8(a)–(d) shows that no obvious external shape was observed between the crystals obtained at 35 or 45°C, but large oblong-shaped crystals that were less in number were observed at 55°C. The particle size distribution of L-alanine crystals produced at different temperatures is presented in Figure 9. The horizontal axis is plotted as a log-scale, and the vertical axis indicates the percentage of cumulative distributions. The average particle sizes of the L-alanine crystals obtained at temperatures of 25, 35, 45, and 55°C were 5.6, 41.3, 44.1, and 57.6 µm, respectively, demonstrating that the particle size significantly increased with the crystallization temperature. Since higher temperatures increase the solubility of the solute, it reasons that the nucleation of L-alanine in solution is retarded at higher temperatures. In addition, higher temperatures facilitate mass transfer of the crystallizing solute, thus leading to continuous crystal growth. As a result, the less nuclei are formed and the preformed nuclei will grow faster, leading to the further growth of individual particles and the production of large crystals (Kim et al., 2014).

SEM photomicrographs of L-alanine crystals obtained using different temperatures: (a) 25°C, (b) 35°C, (c) 45°C, and (d) 55°C. An injection rate of anti-solvent (propanol) of 58.8 mL/min was used.

Particle size distribution of L-alanine crystals produced at temperatures of 25, 35, 45, 55°C. Propanol was injected into L-alanine solutions of 0.01 g/mL with an injection rate of 58.8 mL/min.
Effects of ultrasound power and time Ultrasound is an effective measure for the reduction of both the nucleation induction time and the width of the metastable zone during the crystallization process (Li et al., 2006). Effects of different ultrasound powers (5, 10, and 15 W) and sonication times (10, 30, 60, and 90 s) on the particle size of L-alanine crystals were investigated.
The experiments in this study employed propanol as anti-solvent injected at a rate of 58.8 mL/min at 25°C. Figure 10 depicts the significant morphology differences between raw material and crystalized L-alanine with and without ultrasound. In the presence of ultrasound, crystals of L-alanine appeared as tiny needles that exhibited greatly reduced particle sizes. The effects of ultrasound power and times on volume cumulative distribution are presented in Figure 11(a) and (b), respectively. Average particle size of L-alanine decreased from 25.6 to 9.0 µm with increased ultrasound power, and an extended ultrasound time of 90 s further diminished the crystal size to 5.9 µm.

SEM photomicrographs of L-alanine crystals of (a) raw material, (b) crystalized sample without ultrasound treatment, and (c) crystallized sample with ultrasound treatment (5 W for 30 s). An injection rate of anti-solvent (propanol) of 58.8 mL/min was used.
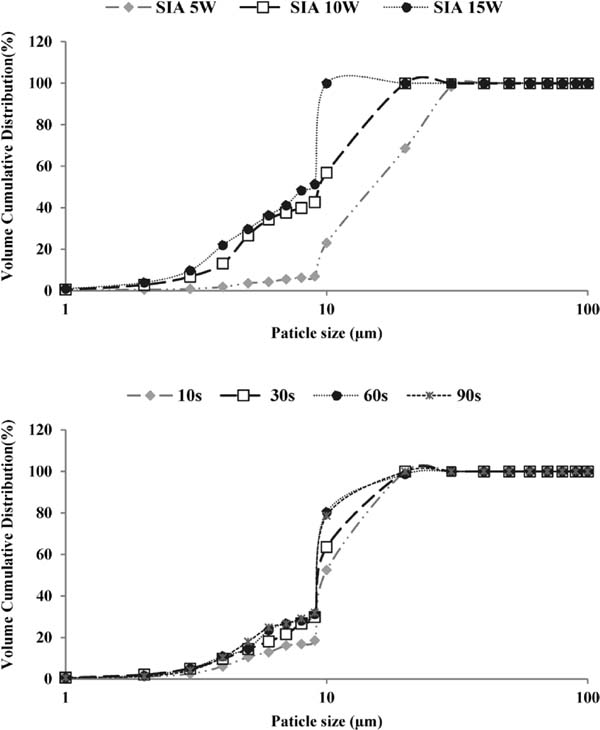
Particle size distribution of L-alanine crystals using (a) different ultrasound powers (5, 10, and 15 W) and (b) ultrasound time (10, 30, 60, and 90 s). L-Alanine solution is injected into propanol (SIA) at an injection rate of 58. mL/min at 25°C.
Similar results have been recorded in other crystallization processes for various materials using ultrasound as an assisted method (de Castro and Priego-Capote 2007; Patel and Murthy 2009, 2011; Ramisetty et al., 2013; Samaram et al., 2014). Ultrasound could cause a higher degree of supersaturation in the target solution, which facilitates the production of more nuclei and thus smaller-sized crystals. In addition, high ultrasound frequency could inhibit the agglomeration of crystals, which brings a higher degree of supersaturation as well as an increased number of crystals, therefore, leading to a reduced particle size (Li et al., 2006, Lyer and Gogate, 2017). Guo et al. (2005) explained this phenomenon as the crystal size was decreased by shock waves (caused by cavitation) and abrasion between crystals, resulting in a reduction in particle size after introducing the ultrasound. Tian et al. (2007) confirmed that sonication time played an important role in the determination of particle size of generated crystals, and glycine solution with longer sonication treatment could get more crystal nucleus, reducing its average grain size of crystals.
In the present study, liquid anti-solvents were employed to produce fine crystals of L-alanine with smaller particle sizes and higher levels of purity. Different organic solvents including methanol, ethanol, and propanol were tested as anti-solvents, and the effects of other process variables such as L-alanine concentration, crystallization temperature, injection rate and method, and ultrasound power and time on particle size of L-alanine crystals were investigated. Overall, propanol was found to serve as the most appropriate anti-solvent for producing small L-alanine crystals. In addition, particle sizes of L-alanine crystals were observed to increase with the increase of L-alanine concentration, injection rate, and crystallization temperature. Ultrasound also showed significant effects on particle size reduction: L-alanine crystal size decreased with the increase of ultrasound power and time.