2017 Volume 23 Issue 4 Pages 561-565
2017 Volume 23 Issue 4 Pages 561-565
The inactivation effect of carbonation under heating (CH) followed by heating (HT2) in the presence of monoglycerol monocaprate (MC10) or monoglycerol monolaurate (MC12) on Bacillus subtilis spores was investigated. CH (80°C, 5 MPa, 10 min) followed by HT2 (90°C, 10 min) induced an approximately 5 log order of inactivation in the presence of MC10 or MC12 at 0.05% (w/v). MC12 appeared to have a greater effect on increasing the inactivation effect of CH followed by HT2 than MC10. Heating, pressurization, and pH downshift involved in CH may play important roles in the high inactivation effect of CH followed by HT2. As indicated by increased DAPI stainability, CH in the presence of MC10 forced germination. The results show that heating after CH in the presence of MC10 and MC12 effectively inactivated B. subtilis spores under milder heating conditions than conventional autoclaving.
Bacterial spores show high resistance to physical and chemical agents, and are hardly inactivated by heat treatment below 100°C. Carbonation treatment is carried out by solubilizing carbon dioxide (CO2) under pressure into liquid food. Carbonation treatment with conditions yielding the supercritical CO2 state can effectively inactivate bacterial spores (Watanabe et al., 2003). However, treatment at milder conditions showed a poor inactivation effect against bacterial spores (Noma et al., 2011). Some emulsifiers, including glycerin fatty acid ester (FAE), show bacteriostatic activity against Bacillus spores (Nakayama et al., 2003). Previously, we reported that monoglycerol fatty acid esters, including monoglycerol caprate (MC10) and monoglycerol laurate (MC12), have bacteriostatic activity against Bacillus spores (Klangpetch et al., 2013; Nakai et al., 2014). In addition, carbonation under heating (CH) in the presence of MC10 and MC12 induced inactivation by 3-4 log order of spores of B. subtilis, B. cereus, B. coagulans, and Geobacillus stearothermophilus in nutrient broth (Klangpetch et al., 2013; Nakai et al., 2014).
It is known that sub-lethal heating of Bacillus spores causes their activation and facilitates germination during subsequent cultivation, thereby inducing decreased heat resistance. Inactivation of bacterial spores using two heat treatments is referred to as “intermittent sterilization.” Spores activated by the first heating are incubated for several hours to induce germination, and the germinated spores are easily inactivated by the second heat treatment. We have reported that CH induced germination of bacterial spores suspended in water without subsequent cultivation, as confirmed by the reduction in heat resistance, decrease in optical density, and increase in DAPI stainability (Noma et al., 2011). In addition, we also showed that combined treatment of CH and MC10 decreased the heat resistance of B. subtilis spores, as compared to CH alone (Noma et al., 2015).
In the present study, we investigated the inactivation effect of CH followed by heat treatment against B. subtilis spores in the presence of MC10 and MC12.
(1) Preparation of bacterial spore suspension B. subtilis 168 was kindly provided by Dr. J. Sekiguchi, Shinshu University. Spores were formed following plating on nutrient agar (NA, Difco; BD, Sparks, MD, USA) and incubation at 30°C for about 4 days. After the number of refractive spores appeared to exceed 90% of the bacterial population, confirmed using phase contrast microscopy (BX 50; Olympus Co., Tokyo, Japan), the spores were collected and washed thrice by centrifugation at 7,000×g and 4°C for 10 min in sterile deionized water. Then, 50 µL of a 10 g/L lysozyme solution was added to the spore pellet, and the suspension was incubated at 30°C for 30 min to degrade the vegetative cells. If needed, spores were purified by density-gradient centrifugation using Percoll plus (GE Healthcare, Uppsala, Sweden) after adjusting the pH to 7.0. The spores were washed 4 times by centrifugation at 10,000×g and 4°C for 10 min in sterile deionized water.
(2) Fatty acid esters (FAEs) Monoglycerol fatty acid esters (monoglycerol monocaprate, MC10; monoglycerol monolaurate, MC12) were provided by Taiyo Kagaku Co. (Mie, Japan). Abbreviation, molecular weight, and hydrophile-lipophile balance (HLB) of the FAEs are summarized in Table 1.
| FAE | Abbreviation | Molecular weight | HLB* |
|---|---|---|---|
| Monoglycerol monocaprate | MC10 | 246 | 6.5 |
| Monoglycerol monolaurate | MC12 | 274 | 5.3 |
(3) Carbonation under heating (CH), first heating (HT1), high pressure treatment (HPT), and second heating (HT2) B. subtilis spores were suspended in sterile deionized water to yield an OD650 of 1.5, and each FAE was added to the suspension at a final concentration of either 0.05% (w/v) or 0.2-2.0 mM. The resultant spore concentration was about 3×105 CFU/mL. The suspension was subjected to CH, HT1, and HPT, followed by HT2. CH was carried out at 80°C, 5 MPa for 10 min using a previously described method (Klangpetch et al., 2013). Briefly, CO2 gas was dissolved into the prepared spore suspension in the pressure vessel at 5 MPa with stirring for 10 min. The treatment period did not include the time to reach 5 MPa pressure, about 1 min. HT1 was performed at 80°C, 0.1 MPa for 10 min in the vessel used for CH without introducing CO2 gas. HPT was performed at 80°C, 5 MPa for 10 min using a method similar to CH, except that nitrogen (N2) gas was used instead of CO2. HT2 was performed using a thermal block (MG-2000; Tokyo Rikakikai Co., Ltd., Tokyo, Japan) at 90°C, 0.1 MPa for 5 or 10 min within 30 min after CH, HT1, and HPT.
(4) Determination of viable spores and inactivation effect The spore suspension was diluted with sterile water, and plated onto NA. After incubation at 30°C for 24 h, the colonies formed were counted. The viable spore count was calculated by multiplying the dilution ratio with the colony count. The inactivation effect was calculated using the following equation:
Log inactivation effect = Log (N0/N), where N0 and N are the initial viable spore count and the viable spore count after each treatment, respectively.
(5) DAPI staining Spores subjected to HT1, HPT, and CH were stained by 4′,6-diamidino-2-phenylindole (DAPI; Nacalai Tesque, Inc., Kyoto, Japan) according to a previously described method (Noma et al., 2011).
(6) Statistical analyses Each experiment was performed three-six times, and the data presented were the average of each data ± standard deviation of the replicated experiments. Significant differences among data were determined by Tukey-Kramer's test (p < 0.05) and a Student's or Welch's t test (p < 0.05).
(7) Experimental design The experimental design of this study is shown in Fig. 1. Viable spores were counted after CH, HT1, HPT, HT2, and their combination treatments. DAPI staining was performed after CH, HT1, and HPT to estimate the germination of B. subtilis spores. The combination treatments, CH followed by HT2, HT1 followed by HT2, and HPT followed by HT2 are abbreviated as CH-HT2, HT1-HT2, and HPT-HT2, respectively.

Experimental design
(1) Inactivation effects of CH, HT2, and CH-HT2 in the presence of FAEs Figure 2 shows the inactivation effects of CH, HT2, and CH-HT2 in the presence of MC10 and MC12. In the absence of FAEs, CH, HT2, and CH-HT2 yielded about 0.5, 2.0, and 2.2 log-order inactivation effects, respectively. The inactivation effect of CH-HT2 did not significantly differ from that of HT2, suggesting that CH did not affect the heat resistance of B. subtilis spores under this condition. The addition of MC10 and MC12 appeared to enhance the inactivation effect of CH, although there was no significant difference (p = 0.12 and 0.14, respectively). The inactivation effect of HT2 tended to be enhanced by MC10 (p = 0.056) and was significantly increased by MC12. The FAEs induced a remarkably higher inactivation effect of CH-HT2 compared to CH or HT2 alone, indicating that CH reduced the heat resistance of B. subtilis spores in the presence of MC10 and MC12. These results indicated that CH-HT2 in the presence of MC10 and MC12 could induce effective inactivation.

Inactivation effects of CH, HT2, and CH-HT2 in the presence of FAEs at a concentration of 0.05%. CH was performed at 80°C, 5 MPa for 10 min, and HT2 was done at 90°C for 10 min. White, gray, and dark gray bars indicate CH, HT2, and CH-HT2, respectively. The different small letters and large capitals indicate significant difference (p < 0.05) in Tukey-Kramer's test among treatments within same FAE and FAE within same treatment, respectively.
(2) Effects of pressurization and heating on inactivation of B. subtilis spores in the presence of FAEs CH treatment involves application of both pressure and heat. Next, the effects of these factors on inactivation of B. subtilis spores were investigated in the presence of FAEs. Pressurization, HPT, was performed under the same treatment conditions (80°C, 5 MPa for 10 min) as CH, except for using N2 instead of CO2. Heat treatment, HT1, was also carried out at the same temperature and time as CH (Fig. 3). In the absence of FAEs, HPT-HT2 showed the highest inactivation effect of 2.2 log order. As shown in Fig. 2, HT2 alone showed an inactivation effect of 2 log order, indicating that HPT did not decrease the heat resistance of B. subtilis spores in the absence of FAEs. MC10 and MC12 similarly increased the inactivation effects of HT1, HT1-HT2, and HPT-HT2, and moreover, MC10 further enhanced the inactivation effect of HPT compared to MC12. These results indicate that both pressurization and heating contribute to the high inactivation effect of CH-HT2 in the presence of MC10 and MC12.

Inactivation effects of HT1, HPT, HT1-HT2, and HPT-HT2 in the presence of FAEs at a concentration of 0.05%. HT1 was performed at 80°C for 10 min, and HPT was carried out at 80°C, 5 MPa for 10 min. HT2 was done at 90°C for 10 min. Light dotted, gray striped, heavy dotted, and black striped bars indicate HT1 alone, HPT alone, HT1-HT2, and HPT-HT2, respectively. The different small letters and large capitals indicate significant difference (p < 0.05) in Tukey-Kramer's test among treatments within same FAE and FAE within same treatment, respectively.
(3) Comparison of inactivation effects among HT1-HT2, HPT-HT2, and CH-HT2 in the presence of MC10 MC10 enhanced the inactivation effects of the three combination treatments (HT1-HT2, HPT-HT2, and CH-HT2) to a level in which almost all spores were killed, making it difficult to compare these inactivation effects (Figs. 2 and 3). Therefore, their inactivation effects were re-evaluated after reducing the HT2 treatment time from 10 min to 5 min (Fig. 4). HT1-HT2, HPT-HT2, and CH-HT2 showed inactivation of 3.3, 4.2, and 5.4 log order, respectively, demonstrating that CH-HT2 had the highest inactivation effect among the treatments. HT1 is accomplished by heating alone. HPT involves heating and pressurization. CH involves a pH downshift to 3.2, in addition to heating and pressurization. It is suggested that heating, pressurization, and pH downshift during CH were all important for the enhanced inactivation effect of CH-HT2 in the presence of MC10.
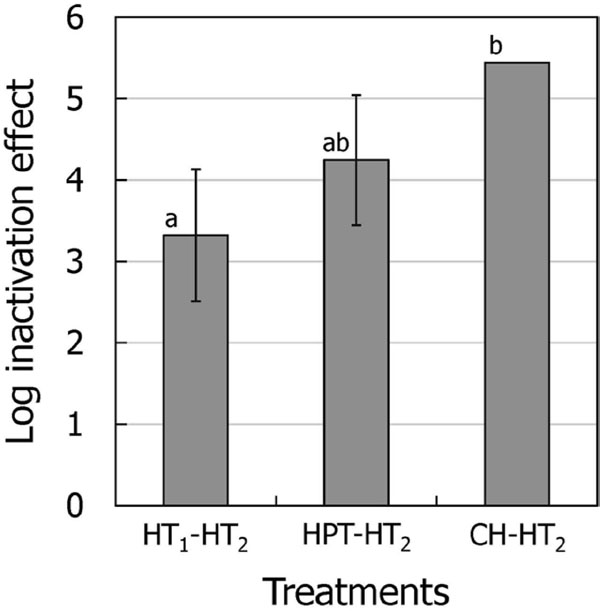
Inactivation effects of HT1-HT2, HPT-HT2, and CH-HT2 in the presence of MC10 at a concentration of 0.05%. HT1 was performed at 80°C for 10 min, HPT was carried out at 80°C, 5 MPa for 10 min, and CH was done at 80°C, 5 MPa for 10 min. HT2 was carried out at 90°C for 5 min. The different characters indicate significant difference (p < 0.05) in Tukey-Kramer's test among treatments.
(4) Comparison between inactivation effects of CH-HT2 with MC10 and MC12 In the experiments shown in Figs. 2–4, inactivation effects were investigated at a FAE concentration of 0.05%. In addition, as demonstrated in Fig. 2, CH-HT2 killed almost all spores in the presence of FAEs. The concentrations of FAEs were set at 0.2–2.0 mM, corresponding to 0.005–0.05% of MC10 for comparing the inactivation effects between MC10 and MC12, and investigating their concentration dependence (Fig. 5). The inactivation effect in the presence of MC12 was significantly higher than that in the presence of MC10 at 0.2 mM (p = 0.031). In addition, MC12 tended to induce higher inactivation of CH-HT2, as compared with MC10 at 0.4 mM (p = 0.090). Therefore, MC12 appeared to have a greater effect on increasing the inactivation effect of CH-HT2 than MC10, although the tendency was limited to 0.2-0.4 mM.

Inactivation effects of CH-HT2 in the presence of FAEs at concentrations of 0.2-2.0 mM. CH was performed at 80°C, 5 MPa for 10 min, and HT2 was done at 90°C for 10 min. Black and white symbols indicate MC10 and MC12, respectively. The symbol “*” indicates significant difference (p < 0.05) in Student or Welch's t test between FAEs within same concentration.
(5) DAPI staining of B. subtilis spores after HT1, HPT, and CH in the presence of MC10 To evaluate the germination level of B. subtilis spores subjected to HT1, HPT, and CH in the presence of MC10, the spores were stained by DAPI and observed under a fluorescence microscope (Fig. 6). Untreated spores were not stained by DAPI, whereas CH induced strong stainability of most spores. HT1 induced DAPI stainability of more spores than HPT, whereas HPT appeared to induce stronger DAPI staining in fewer spores compared to HT1. These results demonstrate that CH effectively causes germination and increases the permeability to DAPI.

DAPI staining of B. subtilis spores subjected to CH, HT1 and HPT in the presence of MC10 at a concentration of 0.05%. CH was performed at 80°C, 5 MPa for 10 min, HT1 was carried out at 80°Cfor 10 min, and HPT was done at 80°C, 5 MPa for 10 min. Scale bars indicate 10 µm.
This paper investigated the inactivation effect of combined treatment of CH and heating (CH-HT2) against B. subtilis spores in the presence of FAEs. The results indicated that CH-HT2 had a higher inactivation effect against B. subtilis spores than CH or heating alone in the presence of MC10 and MC12. Both the pressurization and heating involved in CH seemed to play important roles in this high inactivation effect. In addition, the high inactivation effect may be attributed to germination mediated by CH in the presence of FAEs.
In general, intermittent inactivation treatment is carried out as follows. Spores are heated at sub-lethal conditions for activation, and the activated spores are physiologically germinated during the following cultivation for several hours. The germinated spores are easily inactivated by the second heat treatment. In the present study, we used CH instead of the first heating. During CH, the pH of the spore suspension decreased to about 3.2, making it unlikely that B. subtilis spores will germinate physiologically, as they tend to germinate around neutral pH (Noma et al., 2011). In addition, HT2 was performed within 30 min after CH. We previously reported that CH induces germination of B. subtilis spores in the presence of MC10. Furthermore, the pH of the spore suspension was recovered to about 4.6 just after CH because of the decreased CO2 concentration, and the residual CO2 gas can be easily removed by HT2, suggesting that the enhanced inactivation of spores was not due to HT2 in an acidic condition. Therefore, intermittent inactivation treatment in this study uses physical germination during CH in the presence of MC10. This differs from the conventional intermittent inactivation treatment. The method of this study has the following advantages, as compared to the conventional intermittent inactivation method: (1) a shorter time requirement due to the lack of cultivation for inducing physiological germination, and (2) no unpleasant metabolites produced due to lack of growth of B. subtilis cells. In addition, the downshifted pH during CH can be recovered to almost the original level by the second heating, enabling application of this method to foods with neutral pH.
Monoglycerol FAEs with medium chain lengths, especially monolaurin, show a bacteriostatic effect, that is, they inhibit the growth of bacterial spores (Kimsey et al., 1981). They also increase microbial inactivation effects of other preservation treatments. For example, Fujimoto et al. reported that the inactivation effect of UHT at 125°C for 10 sec in the presence of FAEs corresponds to treatment at 135°C for 30 sec in their absence against G. stearothermophilus spores in chicken extract (Fujimoto et al., 2006). Also, in this study, the inactivation effect of CH followed by heating was higher in the presence of FAEs than in their absence. MC10 and MC12 showed bacteriostatic activity, and their presence at 0.05% completely inhibited the growth of B. subtilis spores (Klangpetch et al., 2013). We have proposed that the bacteriostatic activity of FAEs is raised to inactivation levels by CH (Noma et al., 2015).
HT1-HT2, HPT-HT2, and CH-HT2 treatments in the presence of MC10 inactivated almost all B. subtilis spores (about 5.5 log order). Besides, inactivation effects of HT1-HT2, HPT-HT2, and CH-HT2 in the presence of MC12 were about 3.9, 4.8, and 5.5 log order, respectively. These results indicate that both MC10 and MC12 increased inactivation effects of HT1-HT2 against B. subtilis spores by decreasing heat resistance of the spores. In addition, MC10 and MC12 also enhanced the inactivation of B. subtilis spores by HPT-HT2 and CH-HT2, suggesting that these FAEs decreased the heat resistance of spores under pressure and reduced the pH. Thus, the higher inactivation effect in the presence of MC10 and MC12 can be attributed to the decreased heat resistance of spores, mediated by heating, pressurization, and pH downshift. In addition, MC12 appeared to show higher inactivation effects than MC10 when combined with CH-HT2 (Fig. 5).
The results of DAPI staining of B. subtilis spores indicated that CH induced spore germination in the presence of MC10. Increased dye stainability was one of the germination events that occurred following the reduction in heat resistance. This indicates that spores with increased dye stainability have decreased heat resistance. The result of the CH-mediated increase in DAPI stainability was coincident with the CH-induced decrease in heat resistance. Therefore, the highest decrease in heat resistance by CH in the presence of MC10 may be attributed to the effective induction of germination.
This study demonstrated that heating after CH in the presence of FAEs effectively inactivated B. subtilis spores. Combined treatment of CH and subsequent HT2 can sterilize bacterial spores under milder heating conditions, as compared to conventional autoclaving. Investigation of the effect of food ingredients on the inactivation effect of this method is required. Additionally, evaluation of the quality of foods subjected to this method is also needed.
Acknowledgements This study was supported by JSPS KAKENHI Grant Number 26350094. The authors are grateful to Taiyo Kagaku Co. for providing MC10 and MC12.