2017 Volume 23 Issue 4 Pages 583-593
2017 Volume 23 Issue 4 Pages 583-593
The volatiles of Bacillus subtilis CF-3 showed a significant ( p < 0.05) suppression of the mycelial growth of Monilinia fracticola and Colletotrichum gloeosporioides which caused postharvest decay of Peach and Litchi. An 85-µm polyacrylate (PA) extraction fiber was initially selected used for the optimized experiments. For an optimal CF-3 volatile collection, HS-SPME was suggested to be performed with extraction time of 40.8 min and extraction temperature of 38.5°C and with 41.5 mL LB broth medium and 4.15 mL CF-3 fermentation liquor (1×108 CFU/mL) based on the response surface methodology (RSM). 61 volatile compounds, including fourteen alcohols, fifteen ketones, two aldehydes, eight acids, five phenols, three pyrazines, four esters, three olefins, two amines, two ethers, etc., were extracted using three types of fibers by the optimum method with similarity index (SI)>850 from the database search. Good predictability of the model, satisfactory repeatability for all volatile compounds according to the optimized HS-SPME conditions indicated that the HS-SPME procedure was applicable for the analysis of volatile release, six compounds were selected and assayed for antifungal activity in divided Petri plates, 1-Octanol and 2,4-Di-tert-butylthiophenol can inhibit the mycelial growth of M. fracticola and C. gloeosporioides, which will aid in further research on bio-bacterium volatile metabolites as biocontrol agents against postharvest diseases in fruits and vegetables.
Because the rot of fruits and vegetables after harvest causes great financial losses, an effective approach to reduce the quality losses has become increasingly essential. The commonly used chemical bactericides cause side effects such as pesticide residues, environmental pollution and pathogens resistance to drugs. The severe restrictions on their application will lead to their gradual elimination from the market. Bio-control methods are replacing these chemicals, and these methods not only can control diseases effectively but also are non-toxic to the environment; thus, bio-control methods are increasingly attracting scientific interest (Droby et al., 2009). For example, Pseudomonas fluorescens and Enterobacter cloacae can significantly reduce dry rot severity in potatoes (Al-Mughrabi, 2010). The yeast-like fungus Aureobasidium pullulans PL5 showed potential for use as a microbial antagonist against postharvest fruit pathogens (Banani et al., 2014). To date, several microorganisms and their metabolites have been developed into commercial formulations and are currently used to successfully control diseases in agricultural fields (Kai et al., 2009). The research on bacteria as control agents for postharvest pathogens dates back to 1984 when Pusey and Wilson first demonstrated the potential antagonistic effect of Bacillus subtilis (B. subtilis) application on stone fruit brown rot (Leelasuphakul et al., 2008). Since then, obvious effects by species such as Aspergillus and Penicillium on inhibiting the growth of many phytopathogens have been demonstrated (Foldes et al., 2000, Senthil et al., 2011). B. subtilis is able to operate as an antagonist by the production of antimicrobial substances, including antibiotics, enzymes, lipopeptides, and antifungal volatiles (Chen et al., 2008, Knox et al., 2000, Waewthongrak et al., 2015 and Wu et al., 2005). The separation and purification of some highly efficient bioactive substances (e.g., polymyxin, iturin, chitinase, fengycin) have been performed (Zhang et al., 2013); thus, it is clear that the focus has been on identifying the compositions, structures, and bio-control effects of non-volatile metabolites, while there are very few reports on the volatile metabolites. In some studies, volatile compounds produced by B. subtilis have been shown to have potential antifungal activities; for example, volatiles generated by B. subtilis JA significantly inhibited both spore germination and elongation of germ tubes in Botrytis cinerea using a two-compartment agar-plate assay (Chen et al., 2008). Thus it can be seen that the volatile profile is one of the most typical features of biocontrol bacterium in terms of their bioactivity. Due to the high number of volatile components, the volatile profile will help us to find the key antimicrobial volatile compound and apply to practical production better. However, the research on the diversity, application, and mechanism of antagonism of volatiles generated by B. subtilis is relatively sparse.
The characterization of volatile compounds in biocontrol microorganisms is still a challenge on account of that volatile compounds usually present in microorganisms at very low concentrations, a wide range of polarities, differing solubility, volatility and stability in temperature and pH (D'Agostino et al., 2015). Even so, it is worthy for us to identify and explore the dominant volatiles associated with the biocontrol activity which may have practical use in agriculture. Depending on the species and the level of fermentation, the inhibition rate on pathogens changes due to differences in volatile compound profiles. In previous published works, there is no single optimal method for the collection and identification of volatiles produced by biocontrol bacterium B. subtilis. Ideally, the collection and isolation methods should not discriminate between polar and nonpolar compounds and cause reduction of the volatile compounds or loss of highly volatile compounds (Sawoszczuka et al., 2015). And in volatile profiling, the optimal method should include the maximum number of compounds, normally with different chemical characters and the best response (Azokpota et al., 2009). It follows that the development and validation of a simple and reliable method for collection and determination of volatile compounds in B. subtilis samples is badly in need. Volatiles produced by different types of fungi and bacteria have been collected by several methods, such as solvent extraction, steam distillation, and gas adsorption chromatography, and identified by GC-MS or other authenticating methods. SPME is a novel sample pretreatment and enrichment technology that is widely used in the food, agriculture, pharmaceutical, and environmental fields and in biological matrices (Augusto and Valente, 2002 and Farag et al., 2006). For example, SPME can be used to analyze and identify the aromatic components in food, volatilized substance of blood and herbs, aromatic components in plants, and contaminants in water sources and the environment because of its ease of use and accuracy (Siripatrawan and Harte, 2007 and Zdravkovic, 2015). Use of SPME requires careful attention about various aspects, such as the preparation of samples, extraction temperature and time, the type of extraction fibers. Therefore, establishing a dependable SPME method requires many experiments to optimize and RSM was preferred which is a statistical technique for designing experiments, building models, evaluating the effects of several factors, and identifying optimum conditions, and it has been used to clarify the complex relationships among different variables (Chaichi et al., 2013).
We isolated a strain of bio-control B. subtilis from fermented food during our previous research and registered it as CF-3. CF-3 can strongly inhibit the postharvest pathogenic fungi of fruits and vegetables (Gao et al., 2016). However, the key antifungal volatiles of CF-3 are unclear. On account of preservation method to prolong the fresh-keeping period and maintain the color, flavor and nutrition of fruits and vegetables in maximum had created a huge demand, the optimization of HS-SPME procedure to collect volatiles of B. subtilis will aid the production and application of biocontrol agents in agricultural purposes and lead to steady increase in food preservation and reduce incidence of decay due to postharvest diseases. In the present study, firstly, the biocontrol effectiveness of the volatiles of CF-3 on Monilinia fracticola (M. fracticola) and Colletotrichum gloeosporioides (C. gloeosporioides) which were isolated from infected peach fruit and lychees separately was determined. Secondly, an effective method for collecting the volatiles generated by B. subtilis was developed using a single factor experiment and RSM. Thirdly, we identified the substances obtained from the extraction fibers using GC-MS. To our knowledge, this study represents the prospective application of RSM to optimize HS-SPME conditions for the analysis of volatiles in B. subtilis which will assist to the characterization and establishment of the antifungal nature of organic volatiles produced by bacteria and their potential use in the biocontrol of postharvest diseases in fruits and vegetables.
(1) Bacterial strain and pathogen
B. subtilis CF-3 has been deposited in China Center for Type Culture Collection (CCTCC) and the strain number is CCTCC M 2016125. For short storage, B. subtilis CF-3 was cultivated on Luria Bertani agar (LBA: bacto-tryptone 10 g, yeast extract 5 g, agar 18 g, NaCl 10 g in 1000 mL of distilled water, pH 7.0–7.2) and incubated at 37°C for 24 h. For the volatile compound assays, LB broth (LBB: Bacto-tryptone 10 g/L, yeast extract 5 g/L, NaCl 10 g/L, pH 7.0–7.2) was inoculated with a loop of CF-3 culture followed by culturing 37°C for 24 h with shaking at 150 g in an incubator shaker. Cell suspensions were adjusted to approximately 1 × 108 CFU/mL before using.
The pathogen C. gloeosporioides and M. fructicola were kindly supplied by Prof. Xinchun Zhang of Chinese Academy of Tropical Agricultural Sciences Environment and Plant Protection Institute and Prof. Xiaoyan Zhao of Beijing University of Agriculture (Zhang et al., 2014 and Zhao et al., 2013), separately. They were incubated on potato dextrose agar (PDA: extract of boiled potatoes 200 g, dextrose 20 g and agar 20 g in 1000 mL of distilled water) medium in 9 cm Petri dishes at 28°C for one week prior to use. All chemicals were purchased from Sinopharm Chemical Reagent Co., Ltd (China).
(2) Effect of volatiles of CF-3 on mycelial growth of pathogens
Antagonistic activity of volatiles was tested in sealed plates (Zhao et al., 2011). A 1 cm pathogen agar plug from actively growing mycelium of pathogens was placed centrally on PDA agar test plates. 20 µL quantity of 1 × 108 CFU/mL CF-3 solution was added into the plates containing with LBA and using sterile water as control, coating evenly using spread-plate technique. The bacterial plate was inverted over the PDA plates containing the mycelial plug of pathogens. The compounds identified through GC–MS analysis were purchased (synthetic chemicals) to carry out further antifungal tests in two-grid Petri dishes. Each of the compounds was tested by placing 20 µL singly and used sterile water or solvent aether as the control. Fungal mycelial plugs were placed on the PDA side of the divided plate. Plates were immediately wrapped with parafilm to prevent the escape of the volatiles and incubated at 28°C. The growth of the fungi was measured after 7 days. There were three replicates for each experiment.
(3) Inoculum preparation for HS-SPME
Given that the procedure included an HS-SPME/GC-MS step, it was not possible to analyze the same sample repeatedly; therefore, the experimental plan involved preparing in advance many samples containing a specified volume of CF-3 fermented solution (1 × 108 CFU/mL) and a certain amount of LB broth. The sample volume reflected only the volume of the LB broth; during the trial, the amount of fermented CF-3 solution added to the flask was 10% of the designated volume of LB broth. LB broth blank samples and two empty headspace vials (one at the beginning and the other at the end of the analysis) were also measured in the same conditions to verify that the fiber was clean before conducting the next measurement (Rubio et al., 2014).
(4) Extraction apparatus
A specially designed device is required for providing the proper conditions for both bacterial growth (constant renewal of interior air and removal of produced gases) and easy access for SPME sampling while minimizing losses of volatile substances. Fig. 1 presents the apparatus, including the syringe-like holder and neoteric assembly. We custom-made a 100-mL Erlenmeyer flask that has a plastic cap with threads to ensure a tight seal, and a silica gel gasket was built into the cap to allow convenient insertion of the extraction fibers (Augusto and Valente, 2002). The side arm is bound with eight layers of sterile gauze for aerobic cultivation to provide easy access for oxygen. This custom flask was made by Sanhe Huaxing Experiment Glass Instrument Factory (Haimen, China).
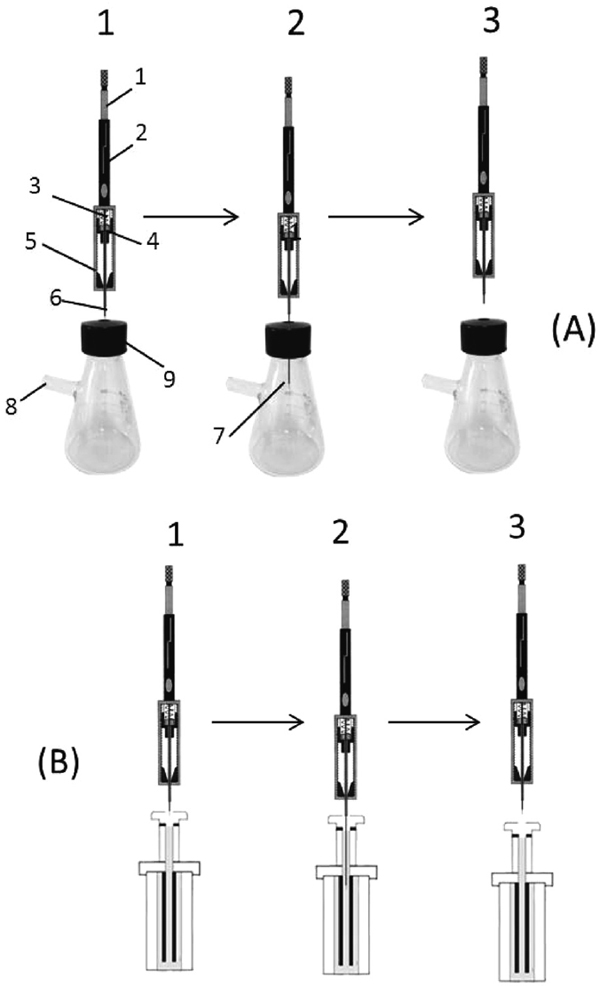
Headspace solid-phase microextraction (HS-SPME) procedure (modified). A=Extraction; B=Desorption. A1=Pierce through septum on sample container; A2=Expose fiber and extract analytes; A3=Retract fiber; B1=Pierce into GC inlet; B2=Expose fiber and desorb analytes; B3=Retract fiber. 1=Plunger; 2=Barrel; 3=Spring; 4=Silicone septa; 5=Holder body; 6=Stainless steel needle; 7=SPME fiber; 8=Side arm with eight layer of gauze; and, 9=Teflon tape seal
(5) Optimization of response surface methodology
Because HS-SPME provides information on the composition of volatile fractions that contribute to the biocontrol activity. To obtain reproducible HS-SPME results, some variables must be controlled during the extraction process. The pre-equilibrium time, extraction time, extraction temperature, salt additives, pH, incubation time, and other factors are important parameters for achieving better extraction efficiency (Adiani et al., 2014). For the sake of minimizing the volatile losses of live samples, these variables include pH, ionic strength were not utilized in the parameters optimization test. And volume of the sample, as well as the extraction time and temperature, pre-equilibrium time and temperature were selected for a single-factor pre-experimental design (Yang & Peppard, 1994). To further determine the settings of the factor levels to be evaluated in the response surface method, the selected three variables based on the most sensitive extraction efficiency were evaluated at four different levels deeply using single-factor experiments: extraction temperature (35°C, 40°C, 45°C, and 50°C), extraction time (30 min, 40 min, 50 min, and 60 min), and sample volume (30 mL, 40 mL, 50 mL, and 60 mL of nutrient solution + 10% fermented bacterium solution). Once sampling was completed, the fiber was withdrawn into the needle and inserted into the GC system port quickly, and the plunger was depressed to begin thermal desorption of the fiber. Samples were analyzed in triplicate. The optimization of the experimental conditions was based on the total peak area and species of volatiles which demonstrated the better experimental level.
RSM has been proposed to evaluate the interactive effects of several factors to determine their influence on a deeper level. Based on the results of the initial one-factor experiment, the levels and effects of three variables, namely, extraction temperature (X1), extraction time (X2), and sample volume (X3), were evaluated. A Box-Behnken design method was used, and a total of 17 treatments were carried out randomly for the optimization procedure. For estimation of the experimental error, the center point was replicated six times. The coefficient determination R2, R2adj, and the prediction error sum of squares (PRESS), which indicate the efficiency of the model's fit, are presented as described by Ferreira (Ferreira et al., 2007). The p-value was used to evaluate the significance of each variable in the model, and additional verification experiments were performed under the optimal conditions to prove the validity of the statistical experimental strategies. The Design-Expert 8.0.5 software package was used to analyze the experimental data and plot the graphs (Mohammadi et al., 2013).
(6) Collection of Volatiles using HS-SPME
The type of fiber was considered as the principal influential factor that interacted with the interrelated parameters during the extraction. During the selection process, more than one extraction fiber should be considered to ensure maximal extraction of all the non-polar, moderately polar, polar volatiles, and semi-volatile substances. Due to the possible loss of important biological volatiles, three fibers were selected for evaluation: fiber of 7-µm polydimethylsiloxane (PDMS, Supelco, Bellefonte, USA) can adsorb nonpolar macromolecular compounds, fiber of 85-µm polyacrylate (PA, Supelco, Bellefonte, USA) prefers polar semi-volatile compounds, and fiber of 100-µm polydimethylsiloxane (PDMS, Supelco, Bellefonte, USA) has a greater range of adsorption of volatile compounds. Before first using the fibers, they were aged in the GC injector according to the manufacturer's instructions to eliminate any possible leftover residue on the coated fiber. We compared the total peak area and the amounts of volatiles between different fibers. Based on the polarity and volatility of the metabolites and considering the limitations of the equipment and the limited service life of the fibers, the fiber with the most complete volatiles among the three tested was considered for further optimization steps for the remaining SPME parameters. Finally, the fiber coated with 85-µm PA was selected for the designed optimizing experiments.
Different amounts of culture medium that contained a certain proportion of the CF-3 cell suspensions were transferred to customized Erlenmeyer flasks. After a 48 h incubation at 37°C with shaking at 150 g, the flasks were placed in a temperature-controlled water bath for 30 min at 30°C to equilibrate, and the flasks were maintained in water baths at different extraction temperatures. Extraction of volatiles from the headspace of the cultures was carried out using the SPME fiber, and various sample amounts, extraction temperatures, and extraction times were configured and executed in the designed scheme. Volatile compounds were desorbed by directly releasing into a heated GC injector.
(7) GC-MS analysis of the volatiles
The volatiles bound to the fiber were desorbed for 5 min in a splitless injector (splitless mode 250°C, fiber penetration depth 55 mm) of an Agilent 6980N gas chromatograph coupled to an Agilent 5975 mass selective detector using a DB-5 ms column (30 m × 0.25 mm i.d. × 0.25 µm, Restek). The carrier gas was helium, which was delivered at a linear velocity of 1 mL/min. The temperature was programmed to be held at 45°C for 5 min, to increase to 120°C at a rate of 10°C/min, to increase to 180°C at a rate of 6°C/min, and finally to increase to 230°C at a rate of 10°C/min and be maintained for 5 min. The mass selective detector was operated in the electron impact ionization mode at 70 eV in a scan range of 45 – 450 m/z. The transfer line and ionization source were thermostatted at 250°C and 230°C, respectively. The volatile compounds were identified by means of their retention times as well as matching the mass spectra with the spectra of reference compounds using the US National Institute for Standards and Technology (NIST, 2008) Mass Spectrometry Library. Determinations were performed by comparing characteristic peaks of the ions. The results of the extraction were evaluated based on the percentages of total volatiles, which were calculated from the peak areas of the total ion current (TIC) profiles. All experiments were performed in triplicate.
The optimum HS-SPME conditions for all target volatiles produced by CF-3 were determined, and these conditions were predicted to result in the most comprehensive extraction based on complex interactions among the SPME variables. This novel method was used with the other two extraction fibers to consider the full range of volatile compounds. The volatiles produced by CF-3 were determined, and experiments were performed in triplicate (Morath et al., 2012).
(8) The determination of dose-response curve of single volatile compound
EC50 was defined as antagonist concentration needed to reduce the fungal mycelial growth by 50% in comparison with the control plates. The values of EC50 were obtained by regression equations where Y represented the pathogen mycelial growth inhibition rate and x is the logarithms of concentration of single volatile compound. 1-Octanol and 2,4-Di-tert-butylthiophenol was procedure by gradient dilution (ten times) from 10 M to 10−5 M and 1 M to 10−6 M separately. The effects on mycelial growth of pathogens were detected. Single compound concentrations and the corresponding inhibitions of mycelial growth were used to calculate EC50 values with the statistical software program log antagonist vs. normalize response-variable slope procedure in GraphPad Prism (version 5.01; San Diego, CA, USA) (Li et al., 2015).
(9) Statistical analysis
Design Expert 8 was used to perform the calculations and prepare the figures for the response surface models. Statistical analyses were performed using SPSS for Windows, version 16.0 (SPSS Inc., 1999). All statistical analyses were performed using one-way analysis of variance (ANOVA) with Duncan's post-hoc test. A significance level of p < 0.05 was used.
(1) Effects of volatiles of CF-3 on mycelial growth of pathogens
In the dual culture assay, the control plates grew actively and spread over the entire PDA plate and in the meanwhile, the diameter treated with volatiles of CF-3 was significantly lower than that in control (Fig.2). The volatile compounds of B. subtilis CF-3 showed strong antifungal activities against mycelial growth of C. gloeosporioides with an inhibition rate of 73.9% ± 2.2% and M. fructicola with an inhibition rate of 59.7% ± 1.2%.
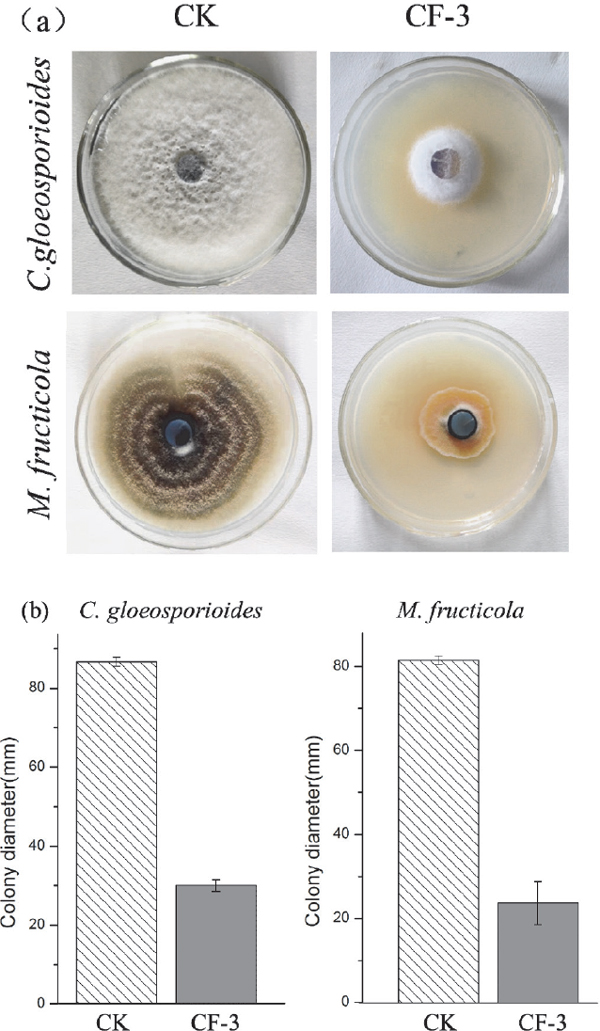
The mycelial growth of C. gloeosporioides and M. fructicola developed in the absence (CK) or in the presence of CF-3. The colony size of each fungus between various treatments was significantly different (p < 0.05).
(2) Optimization of the single-factor experiment and response surface design
After a pre-experimental design, three factors affecting the extraction efficiency (i.e., extraction temperature, extraction time, sample volume) were further investigated in a single-factor experiment. The results under different extraction conditions indicate the best possible central point for the response surface experiments: an extraction time of 40 min, an extraction temperature of 40°C, and a sample volume of 40 mL liquid medium with 10% fermentation broth.
As shown in Table 1, three values, a minimum, a maximum and a central point, coded with −1, 0 and +1 in the experimental design, were tested for each parameter: extraction temperature, 35°C – 45°C; extraction time, 30 – 50 min; and sample volume, 30 – 50 mL. Previous studies on volatile analysis employing HS-SPME have reported desorption temperatures ranging from 250°C to 280°C, with 250°C as the most commonly used setting (Chaichi, Mohammadi and Hashemi 2013, Cheng et al., 2015). Hence, the Box-Behnken design had 17 combinations, including five central point settings, as displayed in Table 2. The response was the sum of the peak areas of the TIC (Total Ion Current for MS responses).
| Parameter | Levels | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| Extraction time/min (X1) | 30 | 40 | 50 |
| Extraction temperature/°C (X2) | 35 | 40 | 45 |
| Sample volume/mL (X3) | 30 | 40 | 50 |
| Standard order | X1 | X2 | X3 | Responses |
|---|---|---|---|---|
| 1 | 0 | −1 | 1 | 1.37E+07 |
| 2 | 0 | 1 | 1 | 1.99E+07 |
| 3a | 0 | 0 | 0 | 1.63E+07 |
| 4 | 1 | 0 | 1 | 1.76E+07 |
| 5 | −1 | 0 | 1 | 1.38E+07 |
| 6a | 0 | 0 | 0 | 2.46E+07 |
| 7a | 0 | 0 | 0 | 1.58E+07 |
| 8a | 0 | 0 | 0 | 1.48E+07 |
| 9 | −1 | 1 | 0 | 1.79E+07 |
| 10 | 0 | 0 | −1 | 1.72E+07 |
| 11a | 0 | 0 | 0 | 1.63E+07 |
| 12 | 0 | 1 | −1 | 2.03E+07 |
| 13 | −1 | −1 | 0 | 1.35E+07 |
| 14 | 1 | −1 | 0 | 1.79E+07 |
| 15 | 0 | −1 | −1 | 1.39E+07 |
| 16 | 1 | 1 | 0 | 1.72E+07 |
| 17 | −1 | 0 | −1 | 1.55E+07 |
Table 3 shows the results from the Box-Behnken experiments using ANOVA and a Fisher F test. The model F-value of 15.17 implies that the model is significant. There is only a 0.08% chance that a model F-value this large could occur due to chance. Values of “Prob > F” less than 0.05 indicate that the model terms are significant. In this case, X3, X1X2, X12, X22, X32 are significant model terms. An analysis of regression was performed to fit the independent variables to a response surface for the experiment. Eqs. (1) illustrates the responses in accordance of the variables. Y is the sum of peak area.
| Source | Sum of squares | d.f.a | Mean square | F valueb | Prob>Fc | Remarks |
|---|---|---|---|---|---|---|
| Model | 3.92E+14 | 9 | 4.35E+13 | 15.17 | 0.0008 | Significant |
| X1 | 5.04E+12 | 1 | 5.04E+12 | 1.76 | 0.2266 | |
| X2 | 8.30E+12 | 1 | 8.30E+12 | 2.89 | 0.1327 | |
| X3 | 1.05E+13 | 1 | 1.05E+13 | 4.65 | 0.0477 | Significant |
| X1X2 | 2.38E+13 | 1 | 2.38E+13 | 8.29 | 0.0237 | Significant |
| X1X3 | 6.55E+12 | 1 | 6.55E+12 | 2.28 | 0.1745 | |
| X2X3 | 1.04E+12 | 1 | 1.04E+12 | 0.36 | 0.5660 | |
| X12 | 1.11E+14 | 1 | 1.11E+14 | 38.56 | 0.0004 | Significant |
| X22 | 1.38E+14 | 1 | 1.38E+14 | 48.11 | 0.0002 | Significant |
| X32 | 5.39E+13 | 1 | 5.39E+13 | 18.79 | 0.0034 | Significant |
| Residual | 2.01E+13 | 7 | 2.87E+12 | |||
| Lack of Fit | 1.45E+13 | 3 | 4.83E+12 | 3.46 | 0.1306 | Not significant |
| Pure Error | 5.58E+12 | 4 | 1.40E+12 | |||
| Cor Total | 4.12E+14 | 16 |
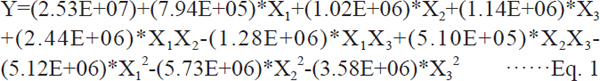 |
Lack of fit F-value is 3.46 which means that the lack of fit is not significant relative to the pure error. There is a 13.06% chance that a lack of fit F-value this large could occur due to chance. This non-significant lack of fit shows that the model is valid for the present study (Januszkiewicz et al. 2008, Pinho et al. 2011). Moreover, the calculated R2pred and R2adj values (0.4153 and 0.8885, respectively) show that the Predicted R-Squared value is not in close proximity to the Adjusted R-Squared value as expected. This may indicate a large block effect or a possible problem with the model with some factors such as model reduction, response transformation to consider (Chauhan and Gupta, 2004). The coefficient of determination R2 value was 0.9512. Put another way, the model could describe 95.12% of the variability in the response. A ratio greater than four is desirable, and the ratio of 10.403 obtained in our study indicates an adequate precision. Thus, this model can be used to navigate the design space. The value for the coefficient of variation (CV) was 9.14%, and a lower CV reflects a better reliability for the experiments. The PRESS, which is usually applied to identify the capability of the proper quadratic model for predictive utilization, was 2.41E+14; this value indicates how the model fits each point in the design. Data were also collected to test the normality of the residuals, as shown in Fig. 3. They demonstrate that the errors are normally distributed and that there are almost no critical violations of the hypothesis based on the analysis. Consequently, the predictive regression model can adequately predict the results obtained from the experimental data.
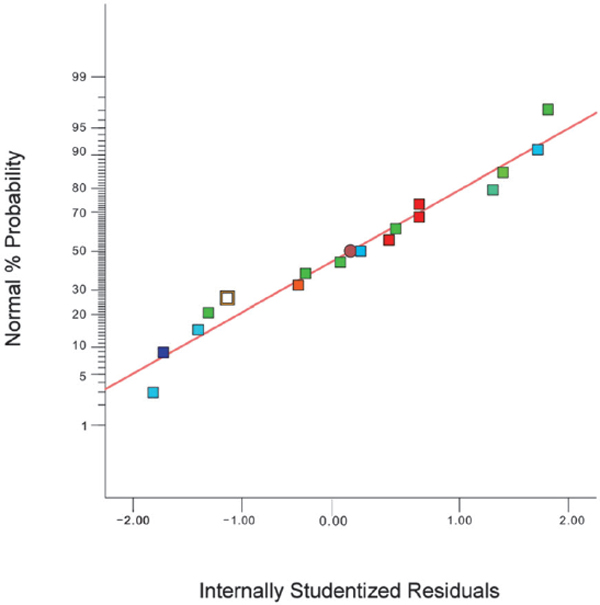
Normal probability plot of residuals for response of total peak areas
Fig. 4 shows the surface plots for the responses of peak areas; the use of three-dimensional plots of the model is strongly recommended in order to evaluate the interactive effect of two variables on a response. When an impact factor was fixed in the extraction conditions, the interaction relationships with the peak areas can be observed, as shown in Fig. 4. The response trend first increased and then decreased, showing that the response surface has a maximum point for the designed experiment. For example, high extraction temperatures have been reported to have an adverse effect on fiber extraction efficiency for low-molecular-weight analytes (Arcanjo et al., 2015, Balasubramanian and Panigrahi, 2011). Fig. 4 (a) shows that in the range of 35°C–45°C, the response for the extraction time increased and then decreased by increasing the temperature. The final adjustment of the factors would yield an optimum extraction method. Based on the above discussion, an intermediate set of conditions was selected, namely, an extraction time of 40.8 min, an extraction temperature of 38.6°C, and a sample volume of 41.5 mL (41.5 mL LB broth and 4.15 mL fermentation CF-3 liquor at 1 × 108 CFU/mL). In accordance with optimized conditions, verification test was performed which showed the peak area of 2.15E +07 with an error that less than 0.05 comparing with the predicting outcomes by model.
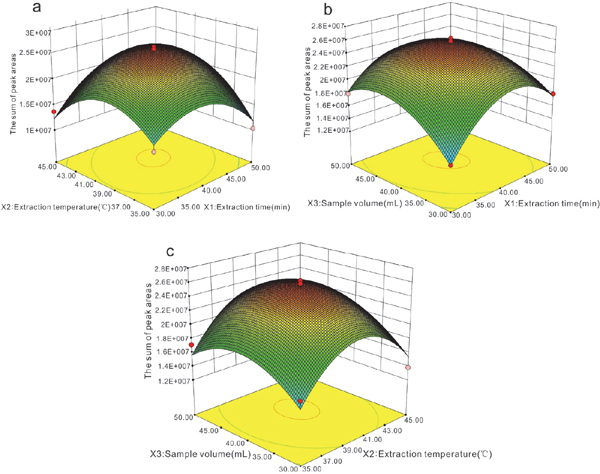
Three-dimensional surface plots of the interaction between different variables: (a) extraction temperature and extraction time; (b) sample volume and extraction time; (c) sample volume and extraction temperature
(3) Identification of the volatiles of CF-3
The accuracy of the method with the 85-µm PA fiber was assessed, and the error of the predicted value of the model was less than 0.05. The model provided credible experimental conditions for an efficient extraction of the volatiles produced by CF-3. The volatiles were extracted and identified using the optimum method measured with three fibers (85-µ m PA, 100-µ m PDMS, 7-µ m PDMS). 61 possible volatile compounds extracted by the different fibers including fourteen alcohols, fifteen ketones, two aldehydes, eight acids, five phenols, three pyrazines, four esters, three olefins, two amines, two ethers, etc (supplementary table 1) (Li et al., 2015, Zheng et al., 2013). Of the volatile compounds assayed for antifungal activity in divided Petri plates, the compounds inhibited mycelial growth of pathogens, suggesting their potential role in biological control. Compared to the spontaneously released metabolites of bacteria which has been reported, some substances which have antifungal activity like 2,4-di-tert-butylphenol (2,4-DTBP) and 1-Octanol, (S)-were not detected in the volatiles of other strains of B. subtilis (Dharni et al., 2014, Chaves-Lopez et al., 2015). The antifungal nature of volatiles has been demonstrated in several pathogen systems, such as inhibition of hyphal extension in Geotrichum candidum by trimethylamine and control of root rot of tobacco by hydrogen cyanide produced by pseudomonads (Robinson et al., 1989, Voisard et al., 1989), the key volatile compounds of CF-3 that play a role in biocontrol effects on postharvest diseases need further research.
(4) Analysis of antifungal activity of detected volatile compounds
On account of limited resources and literature investigation, of the six compounds including 2,4-Di-tert-butylthiophenol, 1-Octanol and so forth were preselected and assayed for antifungal activity in divided Petri plates, 1-Octanol, 2,4-Di-tert-butylthiophenol showed inhibition on the mycelial growth of M. fracticola and C. gloeosporioides, suggesting their potential role in biological control. Accurate estimation of EC50 values relies upon proper analysis of concentration–inhibition data. The dose-response curve of 1-Octanol and 2,4-Di-tert-butylthiophenol was shown in Fig. 5 and Fig. 6. The EC50 value of 1-Octanol on M. fracticola and C. gloeosporioides was 4.77E-005 and 0.97 separately and the 95% confidence intervals were 1.16E-005 to 1.96E-004 and 0.34 to 2.81seperately. The EC50 value of 2,4-Di-tert-butylthiophenol on M. fracticola and C. gloeosporioides was 9.90E-004 and 1.26E-002 separately and the 95% confidence intervals were 2.28E-004 to 4.31E-003 and 4.14E-003 to 3.81E-002 seperately. It should be noted that accurate estimation of EC50 values relies not only on calculation method, but also on fungicide concentration spectrum and the corresponding biological response distribution. At the meanwhile, four substances including 1-Hexadecene, 1,2-Propanediol, Phthalic acid, Dibutyl phthalate had no inhibitory activities to the both fungi. Such antimicrobial activity was evident in different pathogens, making them extremely attractive, in particular, because of their environment friendly. Integration of one or more prevailing strategies to achieve higher levels of disease control and the inclusion of volatiles derived from microorganisms will contribute to an effective commanding of postharvest diseases.
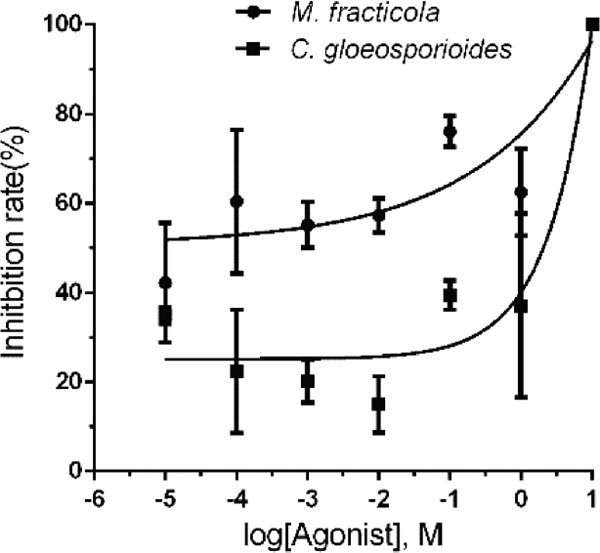
The dose-response curve of 1-Octanol
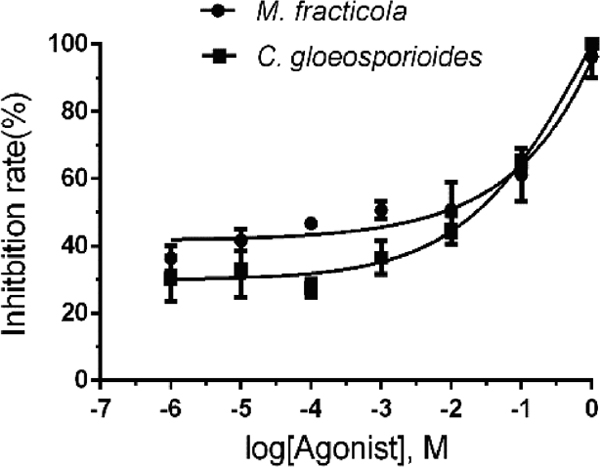
The dose response curve of 2,4-Di-tert-butylthiophenol
Volatile metabolites are an inherent component of the complex functional mechanisms of biocontrol bacteria (Morath, Hung and Bennett, 2012, Raza et al., 2015, Tait et al., 2014). It plays a vital role for biocontrol bacterium to exert bioactivity. Thus, a comprehensive and optimum analytical method for the extraction and identification of the volatiles released by B. subtilis CF-3 is required. The present study describes a powerful approach using an innovative device and an effective experimental design. During the experiments, many difficult problems were solved, such as identifying the correct type of sample bottle. Although B. subtilis CF-3 is an aerobe, it is essential to minimize the loss of volatiles during growth. We designed and customized a triangular flask with a side arm to provide oxygen and used eight layers of gauze to seal the hole of the side arm. A plastic cap was used that contained a built-in silicone pad, which allowed for the insertion of the extraction fiber. This innovative flask did not interfere with the growth of CF-3, but it did allow collection of volatiles generated by CF-3. However, a problem that proved difficult to solve was that for most experiments, triplicate samples were needed. In addition, the influences of bacteria incubation time, extraction fiber service life, and other factors could not be controlled. Considering many factors, we performed the overall optimization with one extraction fiber and then used the optimized conditions for all three extraction fibers to collect the volatiles generated by CF-3.
After obtaining the initial identification results for volatiles from CF-3, additional studies are needed. Results from previous studies and our study indicated that volatiles from B. subtilis have apparent antibacterial effects on pathogens (Chen et al., 2008, Zheng et al., 2013), but which substance plays the key role and the functional inhibition mechanisms of a single substance or all chemical components together are still unknown.
The volatile diversity occurring in microorganisms provides an impressive source of active compounds. In particular, volatiles could play an effective role in an eco-chemical approach in postharvest disease control. Compounds belong to various profiles may show different levels of biocontrol activity. In previous studies, 2-nonanone was identified in the volatiles produced by a Candida intermedia strain used to control postharvest disease in strawberry (Huang et al., 2011), and thymol has been widely used in oat flour as an antimicrobial substance (Sandoval et al., 2011). Furthermore, the research by Zhao et al. demonstrated that in addition to 2-nonanone and thymol, other bioactive volatile compounds, including 2-decanone, 2-methylpyrazine, and β-benzeneethanamine, produced by two isolated B. subtilis strains TB09 and TB32 also have antimicrobial activity (Zhao et al., 2011). Phenethyl alcohol (PEA) has antibacterial activity towards both Gram-negative and Gram-positive bacteria. PEA has bactericidal activity in the concentration of range 90 to 180 mM, and the inhibitory concentration for bacteria is 4- to 5-fold less than the bactericidal concentration (Corre et al., 1990). A comprehensive analysis indicated that 2,4-di-tert-butylphenol (2,4-DTBP) has regulative and control abilities against tomato leaf mold in tomato plants, and 100 µg/mL of 2,4-DTBP can prevent the spore germination of F. oxysporum (Dharni et al., 2014). Furthermore, the proposed mechanism of the antifungal activity of 2,4-DTBP demonstrated that it can both inhibit the assembly of spindle microtubules and disturb chromosomal alignment at the metaphase plate and microtubule kinetochore interactions, which may reduce the germination of spores and mycelial growth (Varsha et al., 2015). Benzoic acid (carboxybenzene) is slightly soluble in water and soluble in ethanol and has apparent inhibition effects on many types of microorganisms, such as yeast, mold, and bacteria. It has thus been widely used as a preservative in food, as have preservatives made from benzoic acid, including benzoate. Additionally, a number of benzoic acid analogs have antifungal activity in in vitro bioassays against strains of Aspergillus flavus, Aspergillus fumigatus, and Aspergillus terreus, which are causative agents of human aspergillosis (Kim et al., 2010). In our study, the bacterial volatiles including 1-Octanol, 2,4-Di-tert-butylthiophenol, showed significant antimicrobial activity. Our results demonstrated that B. subtilis CF-3 apparently possessed a rich source of bioactive volatile compounds. As the literature showed that, Stein et al (2005) revealed that B. subtilis strains can produce more than 24 antifungal substances. The antifungal nature of some of the compounds, such as 2-ethyl-hexanol, 2,4-bis (2-methylpropyl)-phenol, 2-nonanone, have already been demonstrated in several pathogen systems (Fernando, 2005; Almenar, 2007). Future research will be directed toward the efficiency of antifungal volatiles to reduce the decay of postharvest fruits and vegetables. Among the volatiles examined in this investigation, more identification experiments need to be done to discover the effectiveness and feasibility based on their antifungal activity, even different volatile compounds of the same category.
To evaluate the reliability of the proposed method, it was applied to analysis the consistency between predicted and experimented values, and the results declaimed that HS-SPME is a powerful method for monitoring volatile compounds at a very low concentration (Burin V.M., 2013). For most of the volatile compounds the coefficient of variation was below 5%, which verifies the good precision of the method. The achievement of a sensitive and reproducible analytical method that is able to monitor changes in the headspace composition of the flavour sample would be the basis of future research to elucidate the biocontrol potential of volatiles that may be responsible for suppression of postharvest diseases in fruits and vegetables (Moon, 2004). This method does relatively overall the volatile compounds produced by B. subtilis CF-3. Good predictability of the model, satisfactory repeatability for all volatile compounds according to the optimized HS-SPME conditions indicated that the HS-SPME procedure was applicable. However, results of this study show that volatiles of bacteria usually contained more than one kind of antimicrobial compounds. The mixture of compounds produced by B. subtilis may be more effective to control diseases and less likely to select for resistance than treatment by a single compound. The fungistatic effects of identified related single substance or compounding substances are needed to illustrate. Studies are under way to understand the antimicrobial phenomenon of all the different compounds using pure commercial compounds. This study deals with the substantial progress obtained in the use of volatiles as an alternative control measure of fruit and vegetable postharvest diseases, also taking into account constraints and obstacles that hamper their large diffusion and practical application.
The quantity of each substance in the fermentation liquor of B. subtilis CF-3 and their specific fungistatic mechanisms also require identification. These future studies may aid in the identification of new antibacterial substances or reveal antibacterial effects of known substances, which would be valuable to the field of using bacteria metabolites as bio-control agents. The establishment of an effective measurement and evaluation method for maximizing the collection of volatile compounds can help advance the research on and application of volatile antagonistic compounds for the biological control of postharvest diseases in fruits and vegetables. Moreover, the identification of a precursor of a volatile compound that has a good antimicrobial effect can make it possible to easily obtain large amounts of the antagonistic substance through directed biosynthesis through non-genetic manipulation or through the addition of specific exogenous materials to stimulate synthesis of the required metabolites by the bacterium. These approaches provide a vital foundation for the development of biological agents using volatile compounds, which will have long-lasting referential value.
Acknowledgements This study was supported by the National Natural Science Foundation of China (No.31401539), the National Key Technology R&D Program of China (No.2015BAD16B02) and the Project of Food Science Discipline Construction of Shanghai University.
Conflict of interest The authors declare that they have no competing interests.
Ethical approval This article does not contain any studies with human participants or animals.