2017 年 23 巻 5 号 p. 717-723
2017 年 23 巻 5 号 p. 717-723
In this study, we investigated the effects of dietary protein types during late pregnancy on hyaluronan metabolism in the skin of newborn mice. Levels of hyaluronan and hyaluronan synthase (Has)2 mRNA expression increased in the skin of newborn mice from dams fed the gluten diet during pregnancy (Gluten group) when compared with those of newborn mice from dams fed the soy protein diet during late pregnancy (Soy group). In contrast, skin mRNA expression of KIAA1199, which plays a primary role in hyaluronan degradation in the dermis, decreased in the Gluten group. These results show that a maternal low-quality protein diet increases hyaluronan levels, modulates Has2 and KIAA1199 mRNA expression in the skin of newborn mice.
Maternal protein malnutrition can have effects on offspring morphology, physiology and behavior. Variations in the phenotype of the offspring are induced by feeding pregnant dams a diet with a moderate reduction in protein (Yuasa et al., 2016). Some studies have shown that low-protein diets during pregnancy influence body weight, blood pressure and metabolic and intake regulatory systems in offspring (Jahan-Mihan et al., 2015). Furthermore, the protein source in a nutritionally adequate environment influences the characteristics of metabolic syndrome in offspring. Jahan-mihan et al. (2011) demonstrated that body weight, body fat, insulin resistance, and blood pressure were higher in offspring of rats fed a soy protein-based diet compared with a casein-based diet during gestation and lactation. Fetal physiology is sensitive to maternal protein malnutrition during late pregnancy (Gao et al., 2014; Lesage et al., 2002). However, the effects of dietary protein types during late pregnancy on the skin of offspring are not well understood.
Human diets have different protein qualities because animal- and plant-based foods differ in their essential amino acid contents and their digestibility (Pasiakos et al., 2015). High-quality proteins are those that are easily digestible and include essential amino acids in adequate amounts (Han et al., 2015). Soy proteins provide high amounts of essential amino acid that are found in lower concentrations in most cereals (Acevedo-Pacheco et al., 2016). Few studies have revealed that a low-quality protein diet, which includes wheat gluten as the source of protein, can be effective in inducing altered growth (Alippi et al., 2012; Bozzini et al., 2013).
Molecules involved in skin functionality are greatly affected by dietary protein and amino acids. Hyaluronan retains moisture in the skin, facilitates the transport of ion solutes and nutrients, and promotes wound healing. The skin contains the largest concentration of hyaluronan in the body (Laurent and Fraser, 1992). In mammals, hyaluronan is synthesized by isoforms of hyaluronan synthase (HAS), namely, Has1, Has2 and Has3; these synthases have specific properties and are the products of distinct genes that differ in their expression among skin tissues (Weigel et al., 1997). For example, Has2 is the major producer of hyaluronan in the dermis, whereas Has3 is the major hyaluronan producer in the epidermis (Kim et al., 2006). Has1 is the least active, drives the synthesis of high molecular weight hyaluronan and expresses in both dermis and epidermis (Chow et al., 2010). In our previous study, we confirmed that the expression of the HAS2 gene is highly sensitive to the quantity and quality of dietary protein (Oishi et al., 2003). In addition, we showed that deficiency in leucine, cysteine, valine or glutamine tended to decrease hyaluronan synthesis and Has2 transcription (Yamane et al., 2016). The underlying mechanisms are unclear at present but characteristics of proteins including their digestive kinetics, amino acid sequence and bioactive peptides encrypted in their amino acid sequence are potential factors that determine the physiological functions of the skin. Therefore, the aim of the present study was to examine the effects of dietary protein types during pregnancy on hyaluronan metabolism in the skin of newborn mice.
In this study, pregnant C57BL/6 mice were fed an AIN-93G soy protein- or gluten-based diet. The skin levels of hyaluronan and mRNAs involved in hyaluronan synthesis (Has2 and Has3) and hyaluronan degradation (Hyal2 and KIAA1199) in newborn mice were evaluated using ELISA and quantitative PCR (qPCR), respectively.
Materials Soy protein isolate was supplied by Fuji Oil Co., Ltd. (Osaka, Japan). A QnE hyaluronic acid ELISA assay was purchased from Biotech Trading Partners (Encinitas, CA, USA).
Animals and diets C57BL/6 mice (10 – 11 weeks old) that were 15 days pregnant were purchased from Oriental Yeast Co., Ltd. (Tokyo, Japan) and kept in an animal room at 23 – 25°C and 50–56% humidity with a 12-h light cycle (lights on 8:00 – 20:00). Mice had free access to food and drinking water. Animals were randomly divided into the two groups: soy protein [Soy; 2% (w/w) casein + 18% (w/w) soy protein] and gluten [Gluten; 2% (w/w) casein + 18% (w/w) gluten], with two individuals in each group; all animals were fed for 5 days during pregnancy until date of birth (Table 1). Amino acid compositions of experimental diets are shown in Table 2. Within 24 h of birth, all newborn mice were weighed and dissected (8 pups per litter of Soy group; 7 pups per litter of Gluten group). The skin was rapidly frozen in liquid nitrogen, and then used for measuring the level of hyaluronan and various mRNAs. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the Tokyo University of Agriculture. The protocol was approved by the Committee on the Ethics of Animal Experiments of the Tokyo University of Agriculture (Permit Number: 130611).
| Ingredients (g/kg diet) | Groups | |
|---|---|---|
| Soy | Gluten | |
| Cornstarch | 397.5 | 397.5 |
| Casein | 20.0 | 20.0 |
| L-Cystine | 3.0 | 3.0 |
| Sucrose | 100.0 | 100.0 |
| α-Cornstarch | 132.0 | 132.0 |
| Soybean oil | 70.0 | 70.0 |
| Cellulose powder | 50.0 | 50.0 |
| Mineral mixture1 | 35.0 | 35.0 |
| Vitamin mixture2 | 10.0 | 10.0 |
| Choline bitartrate | 2.5 | 2.5 |
| tert-Butylhydroquinone | 0.014 | 0.014 |
| Gluten | – | 180.0 |
| Soy protein | 180.0 | – |
| Groups | ||
|---|---|---|
| Soy | Gluten | |
| Alanine | 3.9 | 3.0 |
| Arginine | 7.2 | 3.9 |
| Aspartate | 11.2 | 4.3 |
| Cystine | 4.2 | 5.1 |
| Glutamate | 20.5 | 39.3 |
| Glycine | 3.9 | 3.7 |
| Histidine | 2.8 | 2.6 |
| Isoleucine | 4.6 | 4.5 |
| Leucine | 8.1 | 7.9 |
| Lysine | 6.3 | 2.7 |
| Methionine | 1.5 | 1.9 |
| Phenylalanine | 5.2 | 5.9 |
| Proline | 6.1 | 15.6 |
| Serine | 5.3 | 5.2 |
| Threonine | 3.9 | 3.1 |
| Tryptophan | 1.3 | 1.1 |
| Tyrosine | 4.0 | 3.8 |
| Valine | 4.7 | 5.0 |
Quantification hyaluronan Skin samples were prepared as described previously (Oishi et al., 2003). Briefly, skin pieces were defatted with acetone, dried, weighed, boiled for 20 min in 50 mM Tris/HCl (pH 7.8) buffer, and then subjected to proteolytic digestion with 1% w/v actinase E for a week at 40°C. Trichloroacetic acid was added to the samples at a final concentration of 10% w/v for deproteinization before centrifugation at 3000 rpm at 4°C for 20 min. Supernatants thus obtained were neutralized with 10 N NaOH. Hyaluronan levels were measured using the QnE hyaluronic acid ELISA, according to the manufacturer's instructions. Properly diluted specimens and hyaluronan reference solutions are incubated in hyaluronic acid binding protein (HABP)-coated microwells. After the removal of unbound molecules by washing, HABP conjugated with horseradish peroxidase solution is added to the microwells to form complexes with bound hyaluronan. Following another washing step, a chromogenic substrate of tetramethylbenzidine and hydrogen peroxide is added to develop a colored reaction. The intensity of the color is measured in optical density units with a spectrophotometer at 450 nm.
RNA extraction and quantitative PCR Total RNA was extracted from the skin using ISOGEN (Nippon Gene Co., Ltd., Tokyo, Japan), according to the manufacturer's instructions. Reverse transcription was performed using a High Capacity cDNA Reverse Transcription Kit under the following conditions: 37°C for 2 h and 85°C for 5 min after preheating at 25°C for 10 min. The amplification products obtained were detected using the THUNDERBIRD™ SYBR® qPCR Mix (Toyobo Co., Osaka, Japan) and StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). Amplifications were performed under the following conditions: 38 cycles of 95°C for 5 s and 60°C for 30 s after preheating at 95°C for 30 s, 95°C for 15 s, and 60°C for 1 min. The relative expression levels of each target gene were calculated using the comparative Ct method using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an internal control. Primer sequences were as follows: GAPDH forward, 5′-CGACTTCAACAGCAACTCCCACT-3′ and GAPDH reverse, 5′-TGGGTGGTCCAGGGTTTCTTACT-3′ (GenBank accession no.: NM_008084); Has2 forward, 5′-GGGAACTCAGACGACGACCTT-3′ and Has2 reverse, 5′-AGATGTACGTGGCCGATTTGT-3′ (GenBank accession no.: NM_008216.3); Has3 forward, 5′-GGATGCCTTGGAGTGAATGTGT-3′ and Has3 reverse, 5′-CAGTCTGCCCAGCAAGTTCTC-3′ (GenBank accession no.: NM_008217.4); hyaluronidase (Hyal)2 forward, 5′-CGGCTCTCTTTCCCTCTGTGTA-3′ and Hyal2 reverse, 5′-CCGAACACGGAAACTGACAAAGT-3′ (GenBank accession no.: AF422177.1); KIAA1199 forward, 5′-AAGAGGCACCACCTTTTCCAT-3′ and KIAA1199 reverse, 5′-GTCTTGGACGTTTGCTTTAGCA-3′ (GenBank accession no.: BC115873.1).
Statistical analysis Results are expressed as means ± SE. Statistically significant differences between the two groups were determined using the Student's t-test. *p < 0.05 and **p < 0.01 indicate values that are significantly different from the Soy group.
Effects of dietary protein types during late pregnancy on body weight of newborn mice Figure 1 shows the body weight of newborn mice. The birth weight of the newborn mice from dams fed the gluten diet during late pregnancy (Gluten group: 1.15 ± 0.01 g) decreased significantly, as compared with newborn mice from dams fed the soy protein diet during late pregnancy (Soy group: 1.26 ± 0.01 g) (Figure 1).
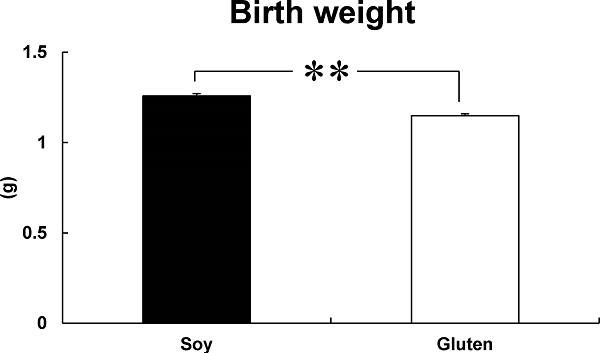
Effect of dietary protein types during pregnancy on body weight of newborn mice. Body weight was measured at birth. Bars represent mean ± SE (n = 14). **p < 0.01 indicates values that are significantly different from the Soy group.
Effects of dietary protein types during late pregnancy on levels of hyaluronan in the skin of newborn mice The effect of dietary protein types during late pregnancy on the skin level of hyaluronan in newborn mice was investigated. Hyaluronan levels in the skin of the Gluten group (1.29 ± 0.08) were higher than those in the skin of the Soy group (1.00 ± 0.04) (Figure 2).
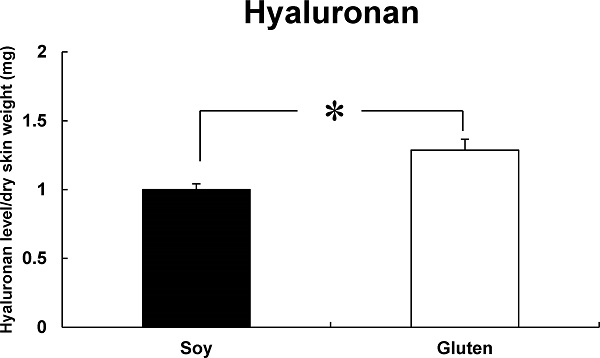
Effect of dietary protein types during pregnancy on the level of hyaluronan in the skin of newborn mice. The amount of hyaluronan in the skin of newborn mice was measured by ELISA. Bars represent mean ± SE (n = 5). *p < 0.05 indicates values that are significantly different from the Soy group.
Effects of dietary protein types during late pregnancy on mRNAs involved in hyaluronan metabolism (Has2, Has3, Hyal2 and KIAA1199) in the skin of newborn mice We investigated the mRNA levels of the 2 major hyaluronan synthases, Has2 and Has3 (Kim et al., 2006). The mRNA levels of Has2 in the Soy group (1.00 ± 0.08) were considerably lower than those in the Gluten group (1.25 ± 0.03) (Figure 3a). No significant differences in mRNA levels of Has3 were observed (Soy group: 1.00 ± 0.01, Gluten group: 1.17 ± 0.11) (Figure 3b). Next, we investigated the mRNA levels of Hyal2 and KIAA1199, which have key roles in degrading hyaluronan (Yoshida et al., 2013). The levels of KIAA1199 mRNA were significantly lower in the Gluten group (0.68 ± 0.02) than in the Soy group (1.00 ± 0.09), but the levels of Hyal2 mRNA did not change (Soy group: 1.00 ± 0.00, Gluten group: 0.90 ± 0.05) (Figure 3c and 3d).
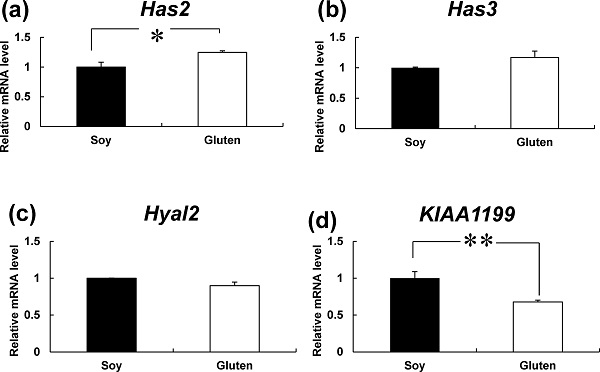
Effect of dietary protein types during pregnancy on mRNAs involved in hyaluronan metabolism (Has2, Has3, Hyal2 and KIAA1199) in the skin of newborn mice. The mRNA levels of Has2 (a), Has3 (b), Hyal2 (c) and KIAA1199 (d) in the skin of newborn mice were measured by qPCR and expressed relative to GAPDH. Bars represent mean ± SE (n = 4). *p < 0.05 and **p < 0.01 indicate values that are significantly different from the Soy group.
Maternal protein malnutrition can affect offspring morphology, physiology and behavior. However, the effects of dietary protein types during pregnancy on the skin of offspring are unclear. The present study examined the effects of a gluten-based diet and a soy protein-based diet during late pregnancy on hyaluronan, which is involved in many biological processes, such as tissue homeostasis and wound healing, and on the regulatory mechanisms responsible for these effects. The results provide the first evidence that dietary protein types during late pregnancy affect hyaluronan metabolism in the skin of newborn mice.
Several studies have reported a decreased birth weight following maternal low quality protein diets (Jansen et al., 1986, 1987). Similarly, the protein source in a nutritionally adequate maternal diet influenced the body weight in offspring (Jahan-Mihan et al., 2011). Jahan-Mihan et al. (2012) have shown that a soy protein-based diet, when compared with a casein-based diet, increased the body weight of the male offspring. Our data showed that the birth weight in the Soy group increased significantly, as compared with the Gluten group. Gluten is known to be a low-quality protein because of lysine deficiency (Rama et al., 1961). It has been shown that different dietary lysine levels influence the growth rates of rats (Ishida et al., 2011). Taken together, it was assumed that the quality of the dietary protein during late pregnancy is a key factor in determining the body weight of the newborn.
Increased hyaluronan synthesis is associated with HAS2 mRNA, the major producer of hyaluronan in the dermis (Spicer and Tien, 2004). The recently described hyaluronan binding/degrading protein, KIAA1199, is expressed in the skin and has key roles in hyaluronan degradation (Yoshida et al., 2013). Our data showed that the hyaluronan levels in the Gluten group increased significantly, as compared with the Soy group. Next, we quantified the levels of skin mRNAs corresponding to key enzymes that are involved in hyaluronan synthesis and hyaluronan degradation. In the present study, Has2 mRNA expression increased significantly and the mRNA levels of KIAA1199 decreased significantly in the Gluten group, as compared with the Soy group. Thus, a gluten diet probably increases hyaluronan levels and modulates Has2 and KIAA1199 mRNA expression. We have previously demonstrated that hyaluronan synthesis was down-regulated by low quality protein diets with wheat gluten in adult rats (Oishi et al., 2003). It is likely that the effects of low quality protein diets on hyaluronan synthesis in the skin of newborn mice are quite different from those in adult mice. We also observed that the mRNA levels of TGF-β1, which enhances Has2 mRNA expression and decreases KIAA1199 mRNA expression in normal human dermal fibroblasts (Nagaoka et al., 2015), were substantially higher in the Gluten group when compared with the Soy group (data not shown). Duarte et al. (32) reported that maternal nutrition affected TGF-β1 gene expression in skeletal muscle of beef cattle fetuses. One reason for the increase in hyaluronan levels may be the increase in TGF-β1 gene expression. The detailed mechanism of upregulation of TGF-β1 expression by gluten diets will be clarified in future studies.
In summary, we showed that maternal low-quality protein diets increase hyaluronan levels and modulate Has2 and KIAA1199 mRNA expression in the skin of newborn mice. Possible explanations for these results include differences in the amino acid composition of proteins. Kabasakal et al. (2015) have suggested that a maternal low-quality protein diet caused differences in plasma amino acid concentrations in weaning rats. Our previous studies have provided the first evidence of the negative effects of an amino acid deficiency on hyaluronan synthesis in human dermal fibroblasts (Yamane et al., 2016). Increased hyaluronan synthesis has been described in a human subject with thickened skin and excessive skin folding (Ramsden et al., 2000). Taken together, our data suggest that the source of protein in pregnant women's diets may influence the skin health of the offspring.
Acknowledgments We would like to thank all of the persons who cooperated in this study. This work was supported by the Fuji Foundation for Protein Research.
Conflict of interest The authors declare no conflicts of interest.