2017 年 23 巻 5 号 p. 725-732
2017 年 23 巻 5 号 p. 725-732
The anti-inflammatory activity of Grifola gargal was evaluated using an in vitro gut inflammation model composed of RAW264.7 and Caco-2 cells. The active compound was isolated and purified to elucidate its structure by instrumental analysis. A low-molecular-weight fraction in G. gargal prepared using an ultrafiltration membrane (13 000 Da molecular-weight cut-off) suppressed tumor necrosis factor-α production and interleukin-8 mRNA expression in an in vitro gut inflammation model. The low-molecular-weight fraction was subjected to sequential extraction, consisting of reverse-phase extraction using 0 – 100% ethanol, in 20% steps, and high-performance liquid chromatography to separate and purify the active compound with anti-inflammatory activity. Instrument analyses indicated that adenine was one of the active compounds. It was suggested that adenine in G. gargal had no small effect on the suppression of inflammatory bowel disease.
Inflammatory bowel disease (IBD), including Crohn's disease (CD) and ulcerative colitis (UC), are characterized by chronic inflammation of the gastrointestinal tract (Baumgart and Carding, 2007; Cho, 2008). IBD causes inflammation of uncertain cause in the intestinal tract. In CD, inflammation frequently occurs anywhere along the digestive tract from the mouth to anus, and also discontinuously in one or more organs. The colon wall is thickened and the inflammation invades from the mucosal membrane to the muscular coat. In UC, inflammation frequently occurs only the large intestine. The colon wall thins and shows inflammation in the mucosal membrane. Inflammation begins in the rectum or sigmoid colon, and spreads up throughout the whole of the large intestine (Bouma et al., 2003). The number of IBD patients in Japan has been increasing mainly in youths (Ishige et al., 2010). Patients who are diagnosed with IBD at a young age are also at increased risk of developing colon cancer later in life (Brackmann et al., 2009; Bergeron et al., 2010; Rudolph et al., 2010).
It has become apparent that intestinal epithelial cells and macrophages secrete large amounts of chemokines and pro-inflammatory cytokines with gastrointestinal tract of IBD patients. Interleukin (IL)-8, a member of the CXC chemokine family, was secreted excessively by a variety of cells, such as intestinal epithelial cells, at the side of inflammation in IBD (Chin et al., 2006). IL-8 causes the excessive recruitment and transmigration of neutrophils into inflamed tissues after injury of the epithelial cells. Lamina propria mononuclear cells from patients with CD spontaneously secreted tumor necrosis factor (TNF)-α and induced epithelial destruction (Zareie et al., 2001). It is well-known that the secretion of inflammatory cytokines such as IL-8 and TNF-α is an important part of the immune response, and the dysregulation of these cytokines is implicated in the pathogenesis of IBD (Gerard and Rollins, 2001).
Treatment of IBD with drugs has mainly focused on steroid medicines. However, these treatments have some serious side-effects (Domènech et al., 2010). Treatment with steroid drugs over a long period frequently induces osteoporosis (weakening of the bones), cataracts, acne, development of a fatty hump at the base of the neck, and a rounded/swollen appearance to the face, diabetes mellitus, and infections. Food factors have also been evaluated to resolve these medically adverse reactions in IBD patients (Hanauer, 2010; Van et al., 2006; Ung et al., 2010). Epigallocatechin gallate (EGCG) and docosahexaenoic acid were reported to suppress IBD by inhibition of both IL-17 and TNF-α expression (Danesi et al., 2010). It was demonstrated that luteolin attenuated dextran sulfate sodium (DSS)-induced colonic injury and inflammation (Nishitani et al., 2013a). Meanwhile, it has been reported that probiotic-induced improvement in murine chronic IBD was associated with the down-regulation of pro-inflammatory cytokines such as IL-6 and interferon (IFN)-γ production in intestinal lamina propria mononuclear cells (Matsumoto et al., 2005). It has also been reported that some basidiomycetes could suppress IBD (Najafzadeh et al., 2007; Førland et al., 2011). It was demonstrated that Grifola frondosa water extract can prevent IBD through suppressing TNF-α production and nuclear factor kappa B (NF-κB) activation (Lee et al., 2010). Nishitani et al. (2013b) also reported that lentinan from Lentinula edodes exhibits intestinal anti-inflammatory activity through the inhibition of IL-8 mRNA expression with TNF receptor 1 endocytosis.
Grifola gargal is an edible mushroom indigenous to Chile and Argentina, and it belongs to the same genus as G. fraodosa. It has been reported that polyphenolic compounds in G. gargal possess anti-oxidant activity (de Bruijn et al., 2009). However, there have been no reports on any other physiological activities in G. gargal. In this study, an in vitro gut inflammation model using the human colon epithelial cell line Caco-2 and the murine macrophage cell line RAW264.7 was used to evaluate anti-inflammatory activity (Tanoue et al., 2008). The anti-inflammatory activity was monitored using suppression of TNF-α production from RAW264.7 cells and IL-8 mRNA expression in Caco-2 cells. The active compound was isolated and purified as the index of this anti-inflammatory activity, and its structure was elucidated.
Materials Actinomycin D, lipopolysaccharide (LPS) from E. coli O127, and TNF-α murine recombinant protein were purchased from Wako Pure Chemical Industries (Osaka, Japan). Dulbecco's modified Eagle's medium (DMEM) with glutamine containing 4.5 g/L glucose and DMEM with glutamine containing 1.0 g/L glucose were purchased also from Wako Pure Chemical Industries (Osaka, Japan). Fetal bovine serum (FBS) was purchased from Daiichi Kagaku (Tokyo, Japan). RPMI 1640 medium, minimum essential medium (MEM) non-essential amino acids (NEAA) and trypsin were purchased from Gibco BRL (Grand Island, USA). Budesonide was obtained from Sigma (St. Louis, USA). G. gargal was provided by the Iwade Research Institute of Mycology Corporation (Mie, Japan).
Fractionation and purification of anti-inflammatory compound in Grifola gargal Hot water extract of G. gargal was prepared as described previously (Harada et al., 2015). Briefly, powdered G. gargal (10 g) in liquid nitrogen was lyophilized and mixed with 200 mL of hot water, and then was heated for 30 min in a boiling water bath with occasional stirring. After centrifugation at 10, 000 rpm for 10 min, supernatants were collected and the precipitates were twice extracted with 200 mL of hot water as described previously. The combined supernatant solutions were lyophilized. The lyophilized product was dissolved in water and was fractioned using an ultrafiltration membrane to exclude molecules with a molecular weight of more than 13, 000 Da. This fraction was successively extracted with hexane, ethyl acetate, and then ethanol. The ethanol fraction was further subjected to reverse-phase extraction using an ODS resin column (2.6 cm × 18.0 cm) and eluted stepwise extract using 0.100% ethanol, with 20% steps. Fractions eluted with 0%, 20%, 40%, 60%, 80%, and 100% were designated “G-ET-1”, “G-ET-2”, “G-ET-3”, “G-ET-4”, “G-ET-5”, and “G-ET-6”, respectively. The active fraction isolated using the ODS resin column was further subjected to high-performance liquid chromatography (HPLC; Hitachi 7000) using a 5C18 PAQ column (4.6 i.d. × 250 mm). The mobile phase consisted of: 10% acetonitrile (0 – 5 min), linear gradient of 10% to 50% acetonitrile (5 – 20 min), 50% acetonitrile (20 – 40 min), linear gradient of 50% to 10% acetonitrile (40 – 55 min) and 10% acetonitrile (55 – 60 min) at a flow rate of 1.0 mL/min, and the effluent was monitored at 210 nm and 260 nm using a UV detector.
Co-culture system Human intestinal epithelial cell line Caco-2 cells (Riken BioResource Center, Tsukuba, Japan) were cultured in DMEM (glutamine, high glucose), supplemented with 1% (v/v) MEM-NEAA, 100 U/mL penicillin, 100 µg/mL streptomycin, and 10% (v/v) decomplemented FBS (56°C, 30 min). Murine macrophage cell line RAW264.7 cells (American Type Culture Collection, Rockville, USA) were cultured in DMEM (glutamine, low glucose) supplemented with 100 U/mL penicillin, and 100 µg/mL streptomycin, and 10% (v/v) decomplemented FBS. Cell cultures were incubated in a humidified 5% CO2 incubator at 37°C. Caco-2 cells were seeded at 3.75×105 cells/ well onto Transwell insert plates (4.67 cm2, 0.4 µm pore size, Corning CoStar Corp., Cambridge, USA). The cell culture medium was changed every 3 days until the cells were fully differentiated (TER value >1 200 Ω·cm2), and the cells were used at passage numbers 48 – 62. RAW264.7 cells were seeded at 8.5×105 cells/well into 6-well tissue culture plates and incubated overnight to completely adhere to the well, and the cells were used at passage numbers 10 – 30. TER value was measured using a Millicell-ERS instrument (Millipore, Eschborn, Germany). After replacing all media with RPMI1640, the Transwell insert on which Caco-2 cells had been cultured was added into multiple plate wells preloaded with RAW264.7 cells. The test sample was dissolved in PBS and mixed in equal amount of 2× RPMI1640 medium. In an experiment to evaluate the anti-inflammatory effect, 0.2 mL of test sample was applied to the apical side for 3 h, and then LPS was added to the basolateral side in this model. After additional incubation of 3 h, the culture supernatants from the basolateral side were collected for TNF-α measurement. Caco-2 cells were harvested for total RNA isolation and applied to subsequent reverse transcription polymerase chain reaction (RT-PCR).
TNF-α content measurement L929 mouse fibrosarcoma cells were purchased from Dainippon Pharma Co., Ltd (Osaka, Japan). TNF-α contents were quantified using a cytolytic assay with L929 cells with murine rTNF-α as the standard, as described by Takada et al. (1994).
RNA isolation and RT-PCR Total RNA was isolated from Caco-2 cells using Sepasol RNA I super (Nacalai Tesque Inc., Kyoto, Japan) in accordance with the manufacturer's protocol. The reverse transcription of the RNA was performed a using High Capacity cDNA Transcription kit (Life Technologies Japan, Tokyo, Japan) in accordance with the manufacturer's protocol. IL-8 and GAPDH primers were form Life Technologies Japan (Tokyo, Japan). RT reactions were performed in a thermal cycler (Gene Amp® PCR System 9700, Applied Biosystems, Tokyo, Japan) at 25°C for 10 min, 37°C for 120 min, and 85°C for 5 min. RT-generated cDNAs were amplified using Taqman Fast Advanced Master Mix (Life Technologies Japan, Tokyo, Japan) in accordance with manufacturer's protocol. To semiquantify the induction of IL-8 mRNA expression in Caco-2 cells, the IL-8 mRNA expression levels were normalized relative to the expression of GAPDH mRNA and IL-8/GAPDH ratios were calculated using densitometric analysis by Image J software. The fold increase in IL-8 mRNA expression was calculated relative to the control. cDNA was amplified using GAPDH and IL-8 primers (Table 1).
| Sense | Anti-sense | |
|---|---|---|
| IL-8 (238 bp) | 5′-TGGCTCTCTTGGCAGCCTTC-3′ | 5′-TGCACCCAGTTTTCCTTGGG-3′ |
| GAPDH (307 bp) | 5′-TGAACGGGAAGCTCACTGG-3′ | 5′-TCCACCACCCTGTTGCTGTA-3′ |
Instrumental analyses In order to separate the active compound, CAPCELL PAK C18 AG (20 i.d. × 250 mm, Shiseido Co., Ltd., Tokyo, Japan) was used as the separation column. The separation was performed using a 600E Multi Solvent System (Nihon Waters K.K., Tokyo, Japan), which was equipped with the 2489 Modular UV/Vis detector. The peaks detected at a detection wavelength of 210 nm were recorded using a Model U-228-1P Unicorder Desktop Recorder (PANTOS CO., LTD., Kyoto, Japan). The fraction with each peak was recovered by concentrating under reduced pressure using an evaporator, followed by freeze-drying. The compounds in the fraction that possessed anti-inflammatory activity was characterized using an Agilent 6510 quadrupole time-of-flight (Q-TOF) mass spectrometer (Agilent Technologies Japan, Ltd., Tokyo, Japan) and JNM-ECX500 (JEOL Ltd., Tokyo, Japan) nuclear magnetic resonance (NMR) spectrometer. To determine adenine contents, ultra performance LC (UPLC) was conducted on an Acquity UPLC H Class system (Waters, Milford, USA) with PDA detector (Waters, Milford, USA). Adenine was identified by the UPLC using a C18 column (Acquity UPLC BEH C18 column, 2.1 × 50 mm, 1.7 µm; Waters, Milford, USA) and a guard column (VanGuard Pre-column, 2.1 × 5 mm, 1.7 µm; Waters, Milford, USA); column temperature, 30°C; mobile phase, solvent A (0.02 M sodium phosphate buffer, pH 6.8), solvent B (methanol); the linear gradient procedure, 0 – 3 min 0% B; 3 – 15 min, 0 – 5% B; flow rate, 0.5 mL/min. The detecting wavelength was set between 200 and 400 nm, and chromatographic peaks were measured at a wavelength of 260 nm.
Statistical analyses Each result was expressed as the mean ± standard error. Statistical significance between more than two groups was analyzed by one-way ANOVA and Tukey–Kramer test.
Purification of an anti-inflammatory compound from Grifola gargal The low-molecular-weight fraction (less than 13, 000 Da) in a hot water extract of G. gargal showed the inhibition of TNF-α production in RAW264.7 cells and IL-8 mRNA expression in Caco-2 cells at the same level of budesonide treatment, which was used as a positive control (Fig. 1). This fraction was subjected to sequential extraction with hexane (G-HE), ethyl acetate (G-EA), and ethanol (G-ET) and residue was collected as G-IS. As shown in Fig. 2, all fractions except G-EA decreased IL-8 mRNA expression levels. The G-ET fraction showed the almost same levels of TNF-α production and IL-8 mRNA expression between 250 and 1, 000 µg/mL as budesonide treatment (Fig. 3). A reversed-phase C18 column was adapted to purify the active compound in the G-ET fraction. G-ET-2 fraction (250 µg/mL), which was eluted with 20% ethanol significantly suppressed IL-8 mRNA expression by approximately 41% compared with LPS treatment in a co-culture system (Fig. 4). Fraction G-ET-2 was subjected to HPLC analysis to further purify the active compound. As shown in Fig. 5, five peaks were detected, and each peak was fractionated to obtain G-ET-2-A, -B, -C, -D and -E in order of retention time. As shown in Fig. 6, any fractions did not show the significant suppressions of TNF-α production and IL-8 mRNA expression. However, fraction G-ET-2-D, for which the retention time was 17.4 min, shows the tendency to decrease TNF-α production and IL-8 mRNA expression as compared to those of LPS treatment (Fig. 6). Since it was assumed that the G-ET-2-D fraction contained an active compound, the G-ET-2-D fraction was collected by HPLC equipped with a separation column.
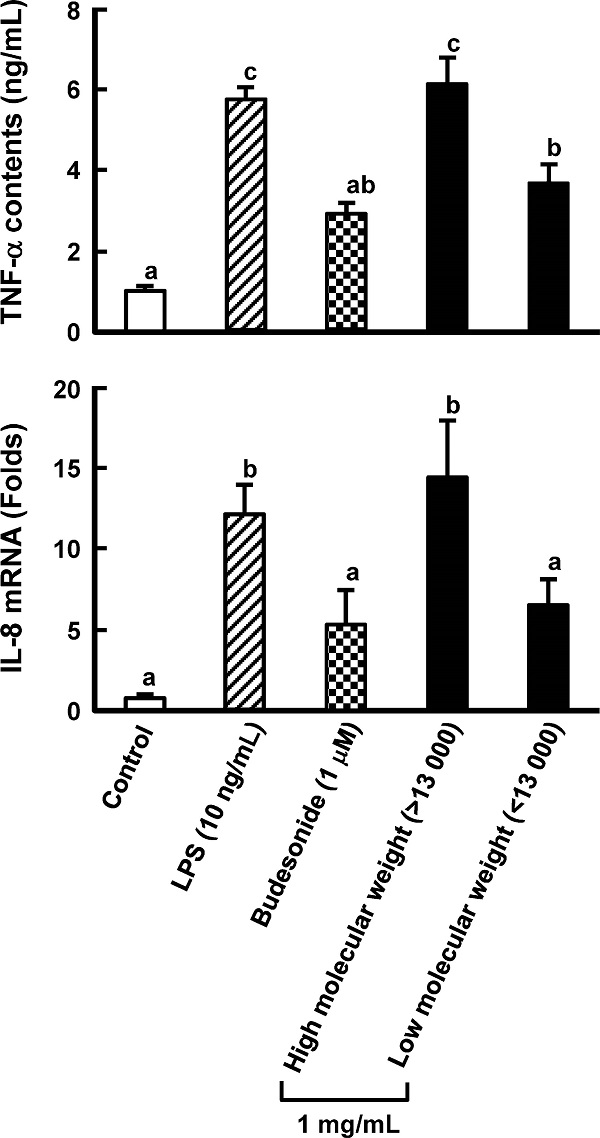
Suppressive effects on TNF-α production and IL-8 mRNA expression by a low-molecular-weight fraction
Each fraction (1, 000 µg/mL) was added to the apical side in the Caco-2/RAW264.7 co-culture model for 3 h. LPS was added to the basolateral side at a final concentration of 10 ng/mL, followed by incubation for an additional 3 h. A) TNF-α secreted from RAW264.7 cells. B) IL-8 mRNA expressed in Caco-2 cells. Values represent the means ± SE (n=3). Items with different letter were significantly different (p < 0.05).

Suppressive effects on TNF-α production and IL-8 mRNA expression by fractions from sequential extraction.
Each sample from sequential extraction (1 000 µg/mL) was added to the apical side in the Caco-2/RAW264.7 co-culture model. Experimental conditions were as described in the Materials and Methods. Values represent the means ± SE (n=3). Items with different letter were significantly different (p < 0.05).

Dose-dependent effect of the G-ET fraction in TNF-α production and IL-8 mRNA expression.
Experimental conditions were as described in the Materials and Methods. Values represent the means ± SE (n=3). Items with different letter were significantly different (p < 0.05).
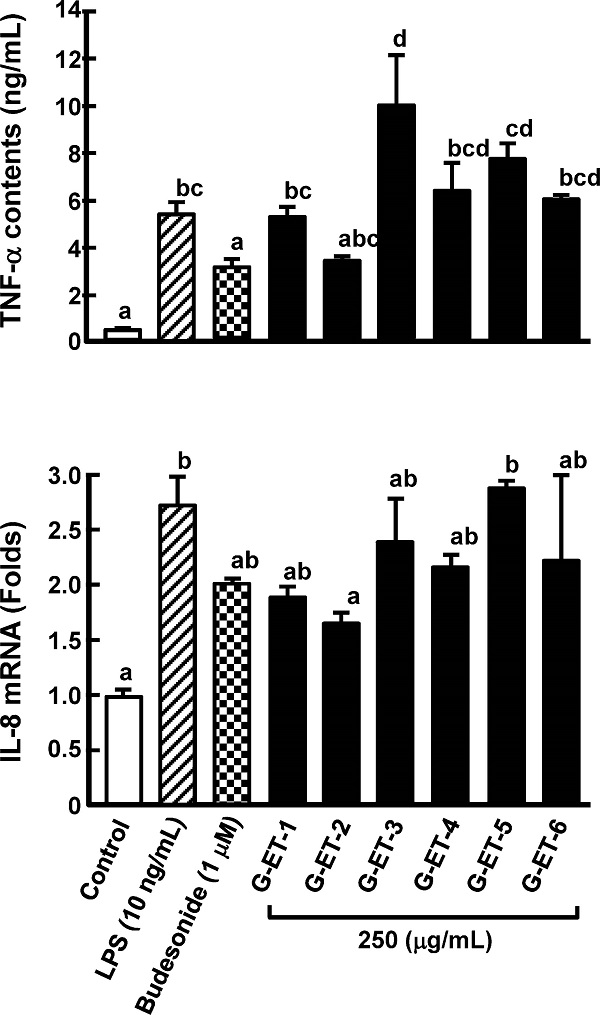
Suppressive effects of each fraction isolated by reverse-phase column chromatography on TNF-α production and IL-8 mRNA expression.
Each sample (250 µg/mL) was added to the apical side in the Caco-2/RAW264.7 co-culture model for 3 h. Experimental conditions were as described in the Materials and Methods. Values represent the means ± SE (n=3). Items with different letter were significantly different (p < 0.05).

High-performance liquid chromatogram of the G-ET-2 fraction.
The G-ET-2 fraction was supplied to HPLC with 5C18 PAQ column using 10% acetonitrile (0 – 5 min), linear gradient scheme 10% to 50% acetonitrile (5 – 20 min), 50% acetonitrile (20 – 40 min), linear gradient scheme 50% to 10% acetonitrile (40 – 55 min), 10% acetonitrile (55 – 60 min), at a flow rate of 1.0 mL/min and the eluted compounds was monitored at 260 nm.
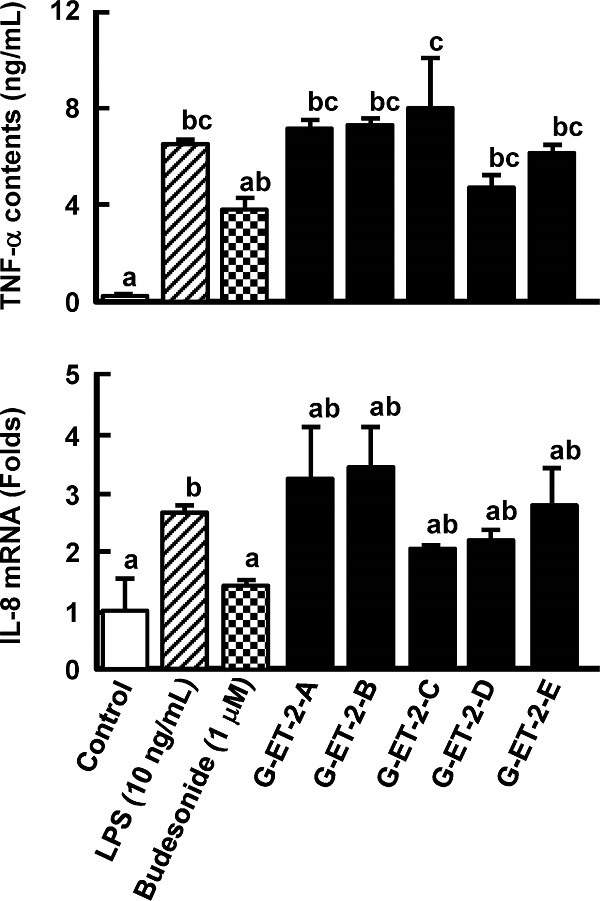
Suppressive effects of each fraction isolated by HPLC on TNF-α production and IL-8 mRNA expression.
G-ET-2 fraction (250 µg) was supplied to HPLC and each fraction was isolated. Each fraction was collected, evaporated and dissolved in 1 mL of RPMI1640 medium. Each fraction was added to the apical side in the Caco-2/RAW264.7 co-culture system for 3 h, and LPS was added into the basolateral side at the final concentration of 10 ng/mL followed by incubation for an additional 3 h. Values represent the means ± SE (n=3). Items with different letter were significantly different (p < 0.05).
Structural analyses of the G-ET-2-D fraction After determining the purification of the G-ET-2-D fraction, its structure was determined by Q-TOF and 1H-NMR analyses. The sample was introduced into the mass analyzer: flow rate: 10 µL/min, ESI positive mode, capillary voltage; 3.5 kV, dry gas temperature; 300°C, dry gas volume; 10 L/min, nebulizer gas; 10 psi, scan range and m/z 50 – 200, and the precise mass of the substance was measured in Scan mode. Next, MS/MS data were measured for the peaks detected in Scan mode, with the collision energy (CE) set to 20 eV. The G-ET-2-D fraction was dissolved in DMSO-d6 and subjected to 1H-NMR. The results of precise mass measurements of the substance demonstrated m/z=136.07425 (within an error of 5 ppm) for MH+, and m/z=158.0429 (MNa+). In addition, from the fragment analysis results, it was estimated that the substance has a molecular formula corresponding to C5H5N5. The 1H-NMR data in DMSO-d6 exhibited signals at δ = 6.910, 8.060, 8.110, and 12.647 ppm (Fig. 7). Based on the database (i), we presumed that the substance showing activity was adenine.
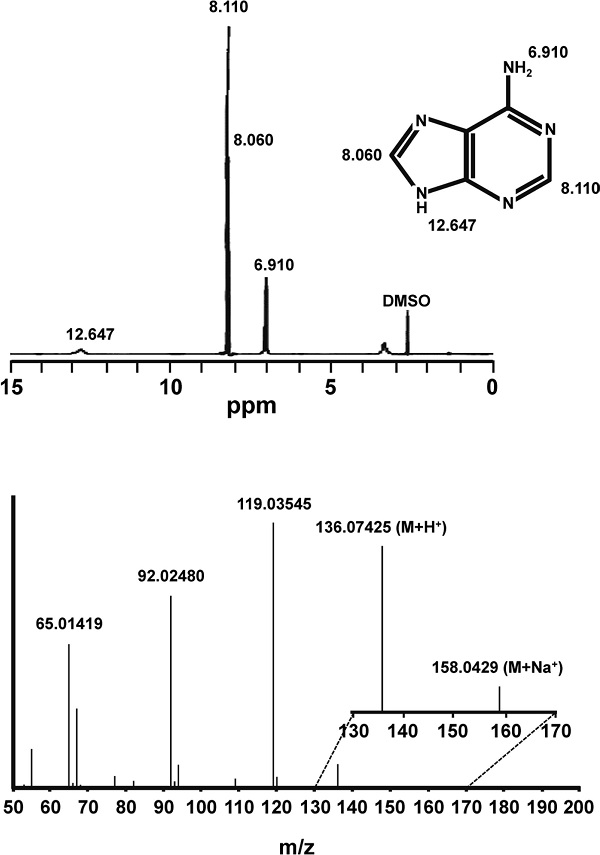
1H-NMR spectroscopy and mass spectrometry of the active compound.
Anti-inflammatory activity of adenine Because adenine was assumed as one of the active compounds with anti-inflammatory activity in G. gargal, the dose-dependent activity of adenine was measured using a co-culture system composed of RAW264.7 and Caco-2 cells. As shown in Fig. 8, it was ascertained that adenine exhibited anti-inflammatory activity at a concentration of 50 µg/mL (330 µM). It was demonstrated that adenine contents in G-ET-2-D fraction prepared using separation column were approximately 32 µg/mL (211 µM) by HPLC analysis (data not shown). This result indicated that the contents of adenine in G-ET-2-D fraction were not sufficient to have anti-inflammatory activity in IL-8 mRNA expression compared with 50 µg/mL authentic adenine, but were adequate to suppress it in TNF-α production. Tanoue et al. (2008) reported that a co-culture system composed of RAW264.7 and Caco-2 cells was useful to screen active compounds with anti-inflammatory activity and IL-8 mRNA expression was affected by TNF-α concentration in basolateral side. Moreover, Nishitani et al. (2013a, 2013b) demonstrated that the compound that shows anti-inflammatory activity in RAW264.7 cells/Caco-2 cells co-culture system attenuated DSS-induced colonic injury and inflammation. Indeed, it has reported recently that adenine reduced IL-8 secretion in Caco-2 cells stimulated with TNF-α and mucosal inflammation in a DSS-induced colitis mouse model (Fukuda et al., 2016). They indicated that administration of adenine at concentration of 5 mg/kg body weight inhibited of weight loss and colon shortening but not at higher concentrations such as 10 and 50 mg/kg body weight. Adenine content in G. gargal was approximately 26.6 mg/100 g fresh weight. It was reported that adenine was present at concentrations of 47.4, 14.9, and 10.6 mg/100 g fresh weight in G. frondosa, Flammuilina velutipes, and L. edodes, respectively (Kaneko et al., 2015). Therefore, it was assumed that edible mushrooms including G. gargal contained enough amount of adenine to show the anticipated efficacy in anti-inflammatory activity. It was well-known that lentinan which was contained in L. edodes possessed anti-inflammatory activity (Nishitani et al., 2013b; Mizuno et al., 2009). The molecular weight of lentinan is approximately average molecular weight 500 000. Because anti-inflammatory activity of G. gargal attributed to low molecular weight fraction, it was clear that polysaccharides in G. gargal do not play a role in its activity.

Dose-dependent effect of authentic adenine on TNF-α production and IL-8 mRNA expression in the co-culture system.
Experimental conditions were as described in the Materials and Methods. Values represent the means ± SE (n=3). Items with different letter were significantly different (p < 0.05).
Acknowledgments This work was supported (in part) by Special Coordination Funds for Promoting Science and Technology, Creation of Innovative Centers for Advanced Interdisciplinary Research Areas, from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Crohn's disease
DMEMDulbecco's modified Eagle's medium
DSSdextran sulfate sodium
EGCGepigallocatechin gallate
FBSfetal bovine serum
HPLChigh-performance liquid chromatography
IBDinflammatory bowel disease
IFN-γinterferon-γ
IL-8interleukin-8
LPSlipopolysaccharide
MEMminimum essential medium
NEAAnon-essential amino acids
NF-κBnuclear factor kappa B
TNF-αtumor necrosis factor-α
UCulcerative colitis