2017 Volume 23 Issue 5 Pages 749-755
2017 Volume 23 Issue 5 Pages 749-755
Some γ-aminobutyric acid (GABA) producing and nitrite reducing lactic acid bacteria were isolated from Sichuan paocai, a Chinese traditional fermented vegetable. Determination of GABA and sodium nitrite residue was carried out by thin layer chromatography combined with HPLC and colorimetric analysis, respectively. It was found that BC114 strain which was identified and designated as Lactobacillus plantarum BC114 possessed the highest GABA producing and nitrite degradation capacity in MRS broth with 5 g/L monosodium glutamate (MSG) and 100 mg/L sodium nitrite. Then, GABA production and nitrite degradation ability were enhanced by optimizing culture conditions, such as time, pH, temperature and MSG concentration. The greatest yield (3.45 g/L) of GABA was determined after 72 h fermentation in MRS with 20 g/L MSG at 30°C and initial pH 5.5. Furthermore, nitrite degradation ability reached the maximum value of 90.17%. These results suggested that L. plantarum BC114 with GABA producing and nitrite reducing ability has the potential to be starter culture and promote the development of functional fermented foods.
Chinese traditional fermentation paocai (CTFP), a type of mildly salted and lactic acid fermented vegetable, is commonly served as a side dish with the main meal or appetizer (Hou et al., 2013). Excess vegetables, such as ginger, cabbage, pepper, cowpea, cucumber and radish, during surplus seasons are usually preserved into CTFP in certain regions of China, especially in Sichuan province. (Zhang et al., 2016). The most current methods of home-based or commercial production of CTFP in Sichuan China are all following the tradition and basing on spontaneous fermentation which is highly dependent on the epiphytic lactic acid bacteria (LAB) which has to be adapted to the intrinsic characteristics of raw vegetables. The fermentation process by LAB may increase the safety, sensory, nutritional and shelf-life properties of vegetable.
As LAB which is the autochthonous microbiota of the raw vegetables play important roles in the traditional fermentation paocai, some LAB strains can be selected and used as starter cultures to enhance other beneficial properties, such as the value-added product of γ-aminobutyric acid (GABA). GABA is a ubiquitous non-protein amino acid and acts as a major inhibitory neurotransmitter in the mammal central nervous system (Schousboe et al., 2007). In addition, GABA is well-known for its physiological functions as follows, antihypertensive effect, antiepileptic effect, antidepressant effect and antidiabetic effect (Li et al., 2010). Owing to these physiological functions, the commercial demand for GABA and GABA-enriched functional foods is increasing with years. The traditional fermented foods such as kimchi (Kang et al., 2016), north-eastern Chinese paocai (Liu et al., 2015), cheese (Diana et al., 2015), Nham (Ratanaburee et al., 2013) and Marcha of Sikkim (Lee et al., 2010) contained GABA. The material basis of beneficial properties is closely linked and dominated by the fermentation strains of the genera Lactobacillus from the old salt brine or the fresh vegetable materials (Anadon et al., 2006). Recently, some of Lactobacillus spp. such as Lactobacillus brevis, Lactobacillus plantarum and Lactococcus fermentum which isolated from traditional fermented food sources has shown the ability to produce GABA (Sasimar et al., 2016). Therefore, the traditional fermentation Sichuan paocai is believed to be a good candidate for the isolation of GABA producing LAB.
In addition to conducting the desirable properties, the spontaneous fermentation of traditional paocai is easily influenced by bacterial contamination which may results in the accumulation of nitrite in products, produce inconsistent and unsafe product. (Zahra et al., 2016). The paocai with high nitrite consumption has been associated with the cancer risk. Extensive experimental and some epidemiological data have suggested that humans are susceptible to carcinogenesis by N-nitroso compounds resulted from endogenous nitrosation reaction of nitrite (Fatemeh et al., 2016). Moreover, Seel (Seel et al., 1994) have found that the high levels of compounds in kimchi after nitrosation may play a role in gastric carcinogenesis in Southwest Korea. To overcome the limitation by nitrite overproduction in paocai associated with spontaneous fermentation, inoculation with starter culture fermentation is currently being investigated. Oh (Oh et al., 2004) have reported that Leuconostoc mesenteroides isolated from Kimchi samples depleted more than 90.0% of 150 mg/L nitrite after 48 h at 30°C. Additionally, several species of LABs isolated from naturally fermented products have been used as starter cultures for controlling fermentation process and enhancing the safety and quality of fermented products. (Liu et al., 2015, Dias et al., 2013). Accordingly, efficient nitrite reducing LAB from the traditional fermentation paocai has also received more attention.
However, so far, there has no studies published about the LAB possessing both GABA producing and nitrite reducing ability. In this study, we screened and evaluated LAB with efficient GABA production and nitrite degradation capacity from traditional fermentation paocai of China, and the both abilities of the excellent LAB strain were increased through optimizing culture medium and conditions. The LAB with the highest GABA producing and nitrite reducing ability can be selected as candidate starter cultures to enhance nutritive and health benefits of the Chinese traditional fermentation paocai.
Culture medium and conditions Lactobacilli MRS broth (Hangzhou Microbial Reagent Co., Ltd, Hangzhou, China) was used for GABA production, nitrite reducing test and maintenance of lactobacillus strains. The LAB strains were incubated in 10 mL MRS broth in universal bottles at 30°C, without shaking. The inoculation size was 1% (v/v) with approximately 8 logs CFU/mL. Monosodium glutamate (MSG) and sodium nitrite (Chengdu Kelong Chemical Co., Ltd. Chengdu, China) were dissolved in distilled water, autoclaved separately and added after sterilization of MRS broth.
Isolation of lactic acid bacteria from Chinese paocai The well-fermented paocai sample was obtained from the traditional markert in Sichuan province of China. Approximately 5 g of paocai sample was suspended in 50 mL of a 1/15 M phosphate buffer solution (pH 6.92) and shaking vigorously for 5 minutes. Suitable decimal dilutions were prepared with phosphate buffer. Then 0.1 mL portions of the diluted sample were plated on MRS medium supplemented with 2% (w/w) agar and followed by incubation at 30°C for 48 h. Totally, 156 colonies were isolated and the colonies were purified by streaking on MRS agar.
Screening of GABA producing LAB by thin layer chromatography (TLC) In order to select the LAB with GABA producing ability, all these colonies were grown in MRS medium containing 10 g/L of MSG at 30°C for 48 h. Then the supernatant was filtered with a 0.45 µm PVDF syringe filter (Merck-Millipore, Billerica, MA, USA) and spotted onto TLC plates (Silica gel 60 F254, Merck, Germany) using the method described by Kim (Kim et al., 2014). The TLC plates were conducted using a solvent mixture of acetic acid: n-butanol: distilled water (2: 3: 5, v/v/v), followed by immersion into a 0.5% ninhydrin solution and developing at 105°C for 10 min.
GABA determinat ion by high performance liquid chromatography (HPLC) For the GABA extraction, an aliquot of MRS medium was centrifuged at 20,000 g for 15 min at 4°C, and then the supernatant was filtered with a 0.45 µm membrane filter. GABA was quantified by HPLC (Waters Alliance 2695, Waters corp., USA) equipped with a fluorescence detector and an Inertsil ODS-3 C18 column (Shimadzu, 4.6*150 mm, 5 um particle). Prior to analysis, the sample was derivatived with the reagent methodology o-phthaldealdehyde (OPA) (Amir et al., 2015). The mobile phases used were A (methanol) and B (sodium acetate (pH6.2): methanol: tetrahydrofuran, 84: 15: 1, v/v/v). The temperature and the flow rate of the elution phase were maintained at 30°C and 1.0 mL/min, respectively (Komatsuzaki et al., 2007). Identification of GABA was performed by comparison to the retention time of GABA standards (Sigma-Aldrich Co., St. Louis, MO, USA), then the GABA yield was calculated based on comparison with standard curves constructed using GABA standards.
Sodium nitrite degradation assay Sodium nitrite degradation ability by screened LAB was measured as described by Wu (Wu et al., 2014). The initial concentration of sodium nitrite in MRS broth was 100 mg/L. Control tube was prepared with sterilized distilled water replacing sodium nitrite. The concentration of sodium nitrite in fermentation broth was determined using colorimetric nitrite assay at 540 nm with a Synergy H4 Multi-Mode Microplate Reader (Bio Tek Instruments, Winooski, VT, USA). The analytical conditions were described by Ito (Ito et al., 1979). The sodium nitrite degradation rate was calculated as the ratio between depletion sodium nitrite concentration and initial sodium nitrite concentration.
Identification of the isolated LAB by 16S rRNA gene sequencing In total, 12 colonies with GABA producing ability were selected and identified by 16S rRNA gene sequencing analysis. The total DNA for molecular identification was extracted according to the instructions of A.E.Z.N.A.TM DNA Kit Protocol (America, OMEGA). Then, the total DNA was used as template for 16S rRNA gene PCR amplification by using the primers Eu27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1490R (5′-GGTTACCTTGTTACGACTT-3′). The thermal cycling conditions were performed as previously reported by Xiang (Xiang et al., 2013). Then the nearly full-length 16S rRNA gene fragment were later sequenced (Invitrogen, ShangHai) and compared with available published sequences in the NCBI database using the BLAST algorithm (www.ncbi.nlm.nih.gov/BLAST/) to identify the strain's closest phylogenetic relatives. Multiple sequence alignment was carried out with CLUSTAL X (Thompson et al., 1997) and the phylogenetic tree was constructed using neighbor-joining method by MEGA 5.0 (Tamura et al., 2007).
Biochemical characterization of the highest GABA producing strain Some biochemical characteristics of the highest GABA producing strain were detected, which included sugar utilization tests, gram staining test, growth temperature and oxygen requirement tests. Fermentation potential of various sugars listed in Table 2 was determined using API 50 CH fermentation strips as follows (Nigatu et al., 2000). The LAB strains were grown in 15 mL of MRS medium at 30°C for 24 h. The cells were collected by centrifugation (5, 000 g for 10 min at 4°C) and then washed twice with PBS. The washed cells were suspended in 2 mL of suspension medium and inoculated into the API 50 CH strips. The strips were incubated at 30°C for 48 h, and then acid formation was determined by monitoring the change in color of bromocresol purple used as an indicator.
| Characteristics | Characteristics | ||
|---|---|---|---|
| Morphology | Arabinose | − | |
| Cell shape | rods | D-xylose | − |
| Gram staining | + | Rhamnose | − |
| Spore formation | − | Mannitol | + |
| Physical growth conditions | Cellobiose | + | |
| Temperature range (°C) | 15–45 | Raffinose | + |
| Anaerobic | + | Fructose | + |
| Aerobic | + | Melezitose | − |
| Sugars utilized | Mannose | + | |
| D-glucose | + | Ribose | − |
| Saccharose | + | Maltose | + |
| Lactose | + | D-sorbitol | + |
Optimization of GABA production and nitrite degradation fermentation conditions The GABA production and nitrite degradation ability by the selected excellent LAB strain was optimized following one factor at a time approach. The strategy was to optimize each parameter independently, such as initial pH (4.0 – 6.5), incubation temperature (20 – 40°C) and incubation.time (0 – 96 h) and MSG concentration (0 – 25 g/L). The citric acid (0.2 M) and sodium bicarbonate (0.1 M) (Chengdu Kelong Chemical Co., Ltd. Chengdu, China) were used to adjust initial pH. Lactobacilli MRS broth was used as a basal medium.
Meanwhile, the growth of Lactobacillus strain was monitored by measuring OD600 with a visible spectrophotometer (Unico WFJ7200, Unico Instrument Co., Ltd. Shanghai, China).
Statistical analysis Values are given as a mean of three independent experiments with ±standard deviation (SD). Results tested for one way analysis of variance (ANOVA) in Microsoft Excel 2010 at p < 0.05 for the determination of significance.
Screening of GABA producing and nitrite degradation LAB strain To select LAB strains from the traditional Sichuan paocai that would produce GABA, the isolates of LAB strains were evaluated using TLC. A total of 12 LAB strains (BC002, BC019, BC110, BC112, BC114, BC120, BC186, BC200, BC201, BC202, BC203 and BC237) were selected from 5 Chinese paocai varieties, and the TLC chromatogram is shown in Fig.1. The mobility spot of culture supernatant of BC114 strain was basically consistent GABA standard and could be preliminary identified as GABA within the margin of error. The measured sample from the fermentation broth which was a complex system may result in incomplete agreement with pure GABA standards. Compared to the other strains, the chromatogram of BC114 exhibited a clear and strong GABA spot. Further, the amount of GABA was quantified by HPLC. Table 1 shows the concentrations of GABA obtained from the 12 strains after 48 h fermentation. The LAB strain BC114 showed the highest GABA generating capacity and reached 1.52 ± 0.07 g/L. Table 1 also depicts the nitrite residue content after fermentation by the selected LAB. The results demonstrated that all the 12 LAB strains showed certain depleting nitrite capacity, compared to spontaneous fermentation. Among the LAB, the strain BC114 was more effective for depleting nitrite and depleted 86.79% nitrite which added to the MRS medium. Based on the above results, the LAB strain BC114 show the efficient performance in the synthesis of GABA and nitrite degradation ability in fermentation medium. LAB are essential for the production of fermented foods, hence, the strain BC114 might be considered as pure culture starter for paocai fermentation with enriched the nutritional value. Therefore, the BC114 strain was selected for the next experimental study.
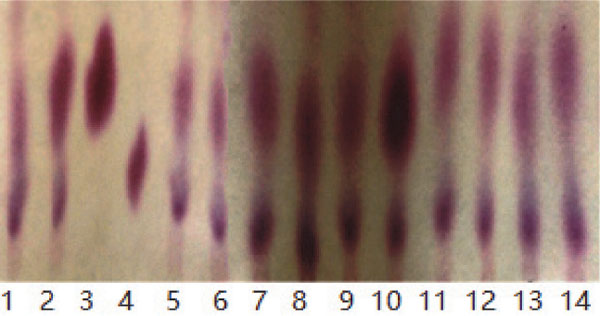
TLC chromatogram analysis of GABA producing ability of selected LAB strains. Lane 3: GABA standard (5 g/L); Lane 4: MSG standard (5 g/L); Lane 1, lane 2 and Lane 5 to Lane 14: The supernatant of fermentation by LAB strains of BC002, BC237, BC202, BC203, BC019, BC110, BC112, BC114, BC120, BC186, BC200 and BC201 in MRS medium with 10 g/L MSG at 30°C for 48 h. The solvent system of TLC was acetic acid: n-butanol: distilled water (2: 3: 5, v/v/v) and the chromatogram was developed in 0.5% ninhydrin solution at 105°C for 10 min.
| Strains | Vegetable source | GABA yield (g/L)D | sodium nitrite degradation rate (%)D |
|---|---|---|---|
| BC002 | Radish paocai | 0.49±0.05 d | 80.74±0.261 m |
| BC019 | Radish paocai | 0.62±0.06 c | 41.36±0.42 b |
| BC110 | Pepper paocai | 0.58±0.08 cd | 61.88±0.665 h |
| BC112 | Cowpea paocai | 0.43±0.01 de | 53.75±0.102 e |
| BC114 | Pepper paocai | 1.52±0.07 a | 86.79±0.36 n |
| BC120 | Radish paocai | 0.45±0.06 de | 48.02±0.65 c |
| BC186 | Cowpea paocai | 0.37±0.008 e | 50.68±0.16 d |
| BC200 | Radish paocai | 0.42±0.03 de | 59.75±0.28 g |
| BC201 | Radish paocai | 0.53±0.04 cd | 58.31±0.75 f |
| BC202 | Ginger paocai | 0.49±0.003 d | 61.36±0.34 h |
| BC203 | Radish paocai | 0.48±0.004 d | 67.32 ± 0.31 k |
| BC237 | Cucumber paocai | 1.12±0.06 b | 65.42±0.087 j |
| Control 1 | 32.80±0.204 a | ||
| Control 2 | 32.50±0.308 a | ||
| Control 3 |
Control 1, the fermentation was carried out in MRS medium containing 100 mg/L NaNO2 without LAB added; Control 2, the fermentation was carried out in MRS medium containing 10 g/L MSG and 100 mg/L NaNO2 without LAB added; Control 3, the fermentation was carried out in MRS medium containing 10 g/L MSG without LAB added.
Identification of GABA producing LAB strain To disclose the microbial taxonomic position and relationships, the phylogenetic tree based on the 16S rRNA gene sequences from the selected GABA producing strain was constructed by neighbor-joining method and was shown in Fig.2. 16S rRNA gene sequence of the highest GABA producing strain BC114 exhibited 100% identity to that of Lactobacillus plantarum JCM 1149 (NR_115605.1), and the sequence was deposited in NCBI under accession no. KU761835. The physiological and biochemical characteristics of strain BC114 are shown in Table 2. The results showed that the pattern of the assimilation sugar characteristics of strain BC114 is similar to the metabolic characteristics of L. plantarum described in the Bergey's Manual of Determination Bacteriology (8th edition). Thus based on the results, the strain BC114 was identified as L. plantarum and designated as L. plantarum BC114.

Phylogenetic tree of the selected 12 GABA producing LAB strains based on 16S rRNA gene sequence
Optimization of GABA production and nitrite degradation fermentation conditions The GABA production and nitrite degradation ability can be improved by adjusting the culture conditions, such as initial pH, fermentation temperature, fermentation time and MSG concentration, which are considered as the most common fermentation factors that affect GABA synthesis or nitrite reducing. Taken this into account, GABA production and nitrite degradation was optimized by measuring the extracellular GABA yield, nitrite residue concentration in L. plantarum BC114 culture at different fermentation time, pH, temperature and MSG concentration in MRS medium.
The fermentation time, pH, temperature and substrate concentration are key factors affecting the metabolites production of microorganism. To consider for GABA synthesis, a time course analysis of L. plantarum BC114 in MRS medium containing 10 g/L MSG and 100 mg/L sodium nitrite with initial pH 6 at 30°C was performed (Fig. 3.A). The results showed that the highest GABA concentration (2.19 ± 0.22 g/L) was obtained at the end of stationary growth phase. The effect of initial medium pH from 4 to 6.5 on the GABA production and growth of L. plantarum BC114 was determined after incubation 72 h (Fig. 3.B). The results showed the enhancement of GABA concentration and cell growth value with increasing pH from 4 to 5.5 and 4 to 6 where the maximum GABA production and cell growth were obtained, following by a reduction of GABA production and cell growth when the initial pH exceeded 5.5 and 6. The maximum GABA production was obtained at pH 5.5. Similarly, Naser (Naser et al., 2015) reported that the favorable initial pH for GABA production by LAB was 5 to 5.5. And Komatsuzaki (Komatsuzaki et al., 2008) have demonstrated an optimal pH value for maintaining the activity of glutamic acid decarboxylase (GAD) which is responsible for the synthesis of the GABA, and the high or low pH may lead to partial loss of GAD activity (Lin et al., 2014). Effect of different culture temperature from 20 to 40°C on GABA production results showed that the optimum temperature was observed at 30°C, and reaching values of 2.72 g/L within a 72 h incubation along with the best cell growth (Fig. 3.C).The temperature was in agreement with the optimum temperature of purified GAD in L. plantarum (Hudec et al., 2015). The optimum medium additive of MSG concentration on GABA production was determined. Fig. 3.D shows the increase of GABA yield with the initial MSG concentration increasing from 0 to 20 g/L where the maximum GABA production was obtained, and followed by a reduction of GABA and OD600 value with the further increasing of MSG. Higher MSG concentration resulted in hyperosmotic stress which will lead to disturbing the bacteria metabolism and the decrease of GABA yield (Komatsuzaki et al., 2008).

Effect of fermentation time (A), initial pH (B), temperature (C) and MSG concentration (D) on GABA production and nitrite degradation rate by L. plantarum BC114 in MRS medium. ▴, GABA yield; ▾, nitrite degradation rate; ɚ, OD600.
Meanwhile, the sodium nitrite degradation ability of the L. plantarum BC114 under these conditions was also analyzed. The fermentation results (Fig. 3) showed that the sodium nitrite degradation rate was not significantly associated with the initial pH, temperature and MSG concentration. While, a study has been demonstrated the nitrite depletion in meats is pH-dependent (Chen et al., 2016). Fig. 3.A showed that the L. plantarum BC114 was very effective for the depletion of sodium nitrite and that mostly nitrite was depleted at the initial stage of 24 h incubation along with the stationary growth phase was reached. With the increasing of incubation time, the depletion rate of sodium nitrite became slow and basically remained a constant value after 48 h. A similar observation on sodium nitrite degradation was also reported by Rao (Rao et al., 2013).
Moreover, under the optimum conditions of MSG concentration 20 g/L, fermentation temperature 30°C, initial pH 5.5 and fermentation time of 72 h, the greatest GABA yield (3.45 g/L) was determined. Furthermore, the sodium nitrite degradation rate reached the maximum value of 90.17%.
Based on the findings obtained from the present study, a LAB strain of L. plantarum BC114 with high capacity of GABA production was isolated from Chinese traditional Sichuan paocai. The optimal of incubation time, fermentation temperature and initial pH and MSG concentration on the GABA production by L. plantarum BC114 with single parameter optimization strategy were further investigated and determined as 72 h, 30°C, 5.5 and 20 g/L, respectively. Furthermore, the L. plantarum BC114 exhibited strong sodium nitrite degradation ability. These results suggest that the isolated LAB strain has the potential to be considered as starter culture in Sichuan paocai industry for production of functional fermented foods.
Acknowledgement This research was supported by grants from the Chunhui Program of Ministry of Education of the People's Republic of China (No. Z2014060), the Applied Basic Research Programs of Science and Technology Department of Sichuan Province (No. 2016JY0253), the Research Fundation of Key Laboratory of Sichuan Province (No. szjj2016-019) and the Transformation of Scientific and Technological Achievements Programs of Sichuan Province (No. 2016CC0074).