2017 Volume 23 Issue 6 Pages 773-782
2017 Volume 23 Issue 6 Pages 773-782
Soy protein has beneficial effects on lowering body weight and blood glucose in patients with diabetes who have overweight or obesity. In addition, soy protein, as complementation medical food material, improves lipid abnormalities such as high triglyceride and cholesterol levels. Kidney disease can cause dyslipidemia, which is considered to be difficult to treat with hypolipidemic agents, statins, and fibrates owing to the impaired renal function. Soy protein improves lipid abnormality in nephropathy patients without concomitant renal function decline. Soy peptide can help with the palliation of pain and improve cytokine levels in combination with medications in rheumatoid arthritis. Furthermore, soy peptide can help to ameliorate inflammatory bowel disease. Thus, soy protein and peptide not only help prevent disease, but may also serve as a complementation medical food material to assist with the treatment of several diseases and disorders.
Metabolic syndrome is caused by overeating (the excessive continuous ingestion of carbohydrate and lipid), and obesity (resulting from low physical activity and/or energy consumption). Particularly, it is generally accepted that increasing intake of saturated fatty acids and animal proteins and decreasing intake of dietary fiber and vegetable protein contribute to the development of metabolic syndrome (Matsuzawa 2005). The core clinical component of the syndrome is visceral fat (i.e., the accumulation of fat in adipose depots around the organs of the peritoneal cavity), whereas the principal metabolic abnormality is insulin resistance (Matsuzawa 1997 and 2006). Locomotive syndrome refers to the functional decline of locomotive organs with aging due to muscle weakness, arthritis, and osteoporosis; locomotive syndrome is a major factor of “shortening of a healthy life” and “bedridden and need of long-term care.”
Metabolic syndrome and locomotive syndrome will not only reduce the quality of life (QOL) for affected people but also bear the financial burden. Medical expenses in Japan continue to increase, with annual national health expenditures of more than 40 trillion yen and 321,100 yen per person (Ministry of Health, Labour and Welfare, 2016, Fig. 1). Improving citizen awareness to prevention and/or treatment of metabolic syndrome and locomotive syndrome is thought to be important for improving QOL for individuals and reducing medical expenses.
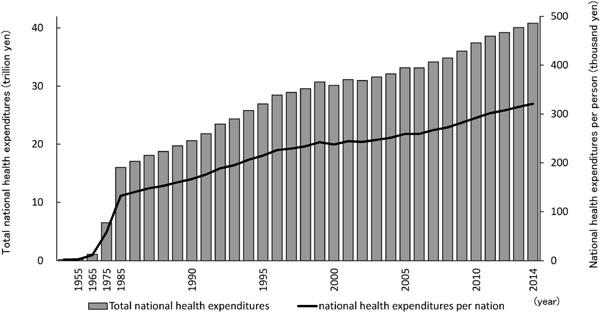
Transition of medical expenses in Japan (drawn from “Overview of national medical expenses” table 1, 2014, Ministry of Health, Labour and Welfare).
Bar: total national health expenditures (left vertical axis, Japanese yen × 1012) and line: national health expenditures per person (right vertical axis; Japanese yen × 1000).
Soy protein exerts not only conventional nutritional value but also beneficial effects on human health. Particularly, soy protein has been reported to help prevent metabolic syndrome and improve various pathological conditions associated with metabolic syndrome (Anderson et al., 1995; Zhan and Ho, 2005; Sirtori et al., 2007). The US Food and Drug Administration (FDA) has approved for food labeling the health claim that the consumption of 25 g soy protein per day reduces the risk of heart disease (Food and Drug Administration. 1999). In Japan, the Consumer Affairs Agency (formerly Ministry of Health, Labour and Welfare) has allowed the health labeling of soy protein as a food for specified health use “to people who are concerned about cholesterol level” (FOSHU, http://www.caa.go.jp/foods/index4.html#m02).
Soy peptide, “Hinute AM” is a food ingredient that was developed with the objective of high absorption of amino acids. Proteins have been thought to be digested (hydrolyzed) by the stomach and intestines and then absorbed as individual amino acids. However, subsequent studies have also revealed the presence of a peptide transporter (Shimizu and Son 2007). In order to increase protein absorptivity, it is better to hydrolyze proteins into a mixture of amino acids and di- and/or tri-peptides before consumption. Dipeptides, tripeptides, and free amino acids account for 60% or more of the total nitrogen in Hinute AM, and the high amino acid absorption efficiency of Hinute AM has been clinically studied (Maebuchi et al., 2007). Many beneficial effects of Hinute AM on human health have been reported (Masuda et al., 2007; Matsushita et al., 2012; de Oliveira et al., 2015; Imai et al., 2017).
In this review, the author introduces soy protein and soy peptide as complementation medical food materials for the prevention of metabolic syndrome and locomotive syndrome, as well as for improving dyslipidemia and rheumatoid arthritis symptoms.
Background Statins and fibrates are drugs developed to improve blood lipid levels. Statins are known as the most efficient agents for reducing plasma cholesterol. Statins target hepatocytes and inhibit 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, the enzyme that converts HMG-CoA into mevalonic acid, a cholesterol precursor. The inhibition of HMG-CoA reductase decreases intracellular cholesterol levels, inducing the activation of translocating of sterol regulatory element binding proteins (SREBPs) from the endoplasmic reticulum to the nucleus. In the nucleus, SREBPs increase gene expression for the low-density lipoprotein (LDL) receptor. The reduction of cholesterol in hepatocytes leads to an increase of hepatic LDL receptors, which help reduce circulating LDL and its precursors (intermediate density and very low-density lipoproteins [VLDL]), thus resulting in significant reductions in plasma LDL-cholesterol levels (Stancu and Sima 2001; Vrablik and Ceska 2015).
Fibrates, which are antagonists of peroxisome proliferators-activated receptor α (PPARα), are used in accessory therapy in many forms of hypercholesterolemia, usually in combination with statins. Although less effective in lowering LDL levels, fibrates increase HDL, lower triglyceride levels, and reduce insulin resistance. When PPARα is activated by fibrates, β-oxidation of fatty acids proceeds, and the synthesis of triglycerides and the secretion of VLDL from hepatocytes lower, resulting in significant reductions of plasma triglyceride levels. The enhanced catabolism of VLDL generates surface remnants, which are transferred to HDL. HDL concentrations are further augmented by an increase in PPARα-mediated transcription of apolipoprotein AI and apolipoprotein AII. Thus, plasma HDL-cholesterol levels increase significantly. (Tenenbaum and Fisman, 2012).
There are known serious side effects from statins, including muscle symptoms, rhabdomyolysis (secondary renal failure due to destruction of specific muscle tissue), peripheral neuropathy, myopathy, liver dysfunction, and thrombocytopenia (Laufs et al., 2015; Kwak 2014; Lei et al., 2014; Haque et al., 2016). Rhabdomyolysis often induces sudden kidney failure (Ambapkar et al., 2016). Accordingly, it is necessary to carefully consider the pharmacokinetics of myogenic enzymes such as creatinine phosphokinase (CPK) and myoglobin at the time of administration of statin. Fibrate-related side effects include the development of slight gastric region discomfort and myopathy (myalgia with the increased CPK). Because fibrates increase cholesterol in the bile duct, the risk of gallstones increases. When statins and fibrates are used in combination, the risk of rhabdomyolysis is reported to rise. Combinations of these two agents are, in principle, contraindicated.
Soy protein lowers serum cholesterol levels by acting as a bile acid sequestrant, which binds bile in the gastrointestinal tract to prevent its reabsorption by performing the same anion exchange reaction as the resin cholestyramine (Nakamura and Matsuzawa, 1994). Soy protein removes bile acids from the body by forming insoluble complexes with bile acids in the intestine, which are then excreted in the feces. The loss of bile acids results in higher levels of cholesterol transport into hepatocytes for conversion to bile acids. Consequently, serum cholesterol levels are normalized (Sugano et al., 1988 and 1990, Fig. 2). The mechanism by which soy protein lowers cholesterol differs from that of statins. Accordingly, statin and soy protein are expected to act additively or synergistically to decrease cholesterol levels.

The mechanism by which soy protein and statins lower cholesterol.
Statin inhibits 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductase, and soy protein removes cholesterol-derived bile acids from the body in the feces.
In this review, the author introduces the potential beneficial effect of a soy protein-based snack (low-energy, high-protein, low-carbohydrate, and very low-fat) on dyslipidemia (Nabiki et al., 2016). Moreover, we show an improvement in diabetic nephropathy with a soy protein snack and an effect on chronic kidney disease with soy protein β-conglycinin complementation.
Soy protein for dyslipidemia The Japan Society for the Study of Obesity defines obese individuals as those with a body mass index (BMI) of 25 kg/m2 or higher (Examination committee of criteria for “Obesity diseases' in Japan 2002). Nabiki et al. (2016) examined the effects of a soy protein snack on weight loss, markers of diabetes, and parameters of dyslipidemia in obese diabetic patients with high levels of LDL-cholesterol and triglyceride who were treated with statins and/or fibrates (Table 1, protocol 1). In this study, there were 28 hyperlipidemic patients who did not have an adequate improvement in blood lipids even after continuous ingestion of the lipid-improving drugs (fibrates, 24 patients; statins, two patients; both drugs concomitant medication, two patients). These participants consumed a soy protein snack containing 6.8 g of soy protein (total 17.6 g and 62.4 kcal) per day along with the lipid-improving drugs. They were also instructed to consume 300 mg or less of cholesterol, 50 g or less of fat, and to eat fish products rather than meat products. As a result, these patients experienced a significant decrease in body weight of about 1 kg and a significant decrease in waist circumference of about 2 cm. Total cholesterol, triglyceride, LDL-cholesterol, apolipoprotein B, and glycated hemoglobin levels decreased significantly, and HDL-cholesterol level increased significantly (Fig. 3). In addition, a lipid metabolism-improving effect was also observed in patients who did not lose weight, and this effect was suggested to be a direct effect of soy protein. Therefore, the use of soy protein may help to reduce the drug dose for dyslipidemia (Nabiki et al., 2016).
| Protocol 1 | Protocol 2 | Protocol 3 | Protocol 4 | |
|---|---|---|---|---|
| Study title | Effect of Soy Protein in Dyslipidemia of Lipid Abnormalities Patients | Effect of β-Conglycinin on Hyperlipidemia in Subjects with Renal Dysfunction | Effects of Resistance Training with or without Nutritional Supplementation of Soy Peptide | Effect of Soy Peptide in Joint Symptoms of Rheumatoid Arthritis Patients |
| Design of study | Non-blind open study | Non-blind open study | RCT (Randomized controlled trial) | Placebo-control led double blind study |
| Number of subjects | 28 | 14 | 33 | 16 |
| Duration of study | 1-4 months | 3 months | 12 weeks | 8 (+4) weeks* |
| Dose and mode of administration | 6.8 g Isolated soy protein Protein bar (17.6g/2 pieces) |
4.6 g β-conglycinin Chewable tablets (12.6 g/8 pieces) |
8.0 g Soy peptide Beverage (250mL/1 bottle) |
8.0 g Soy peptide Powdered juice (30g) |
| Primary efficacy parameters | TG, LDL-C, HDL-C, TC, Apo-B, FPG, HbA1c | TG, LDL-C, HDL-C, FPG, HbA1c | Knee-extension power, Walking speed, Dorsiflexion power, Eye opening stand up time | Awareness and objective symptoms (Pain score, VAS, DAS28, CDAI) |
| Secondary efficacy parameters | ALB/Cr | TP, Cr, UA, ALB/Cr | White cell count, AL, Cr | Inflammatory cytokine (TNF-a, IL-1β, IL-6, CRP, MMP-3, ESR) |
TG, triglyceride; LDL-C, LDL-cholesterol; HDL-C, HDL-cholesterol, TC, total-cholesterol; FPG; fasting plasma glucose; ALB/Cre, urinary albumin to creatinine ratio; TP, total protein; Cr, creatinine; UA, uric acid; VAS, visual analog score; DAS28, disease activity score 28; CDAI, clinical disease activity index; TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β; IL-6, interleukine-6; CRP, C-reactive protein; MMP-3, matrix metalloprotease-3; ESR, erythrocyte sedimentation rate.

Serum lipid improvement in participants treated with a combination of a soy protein snack and dyslipidemia medications (modified from Nabiki et al. 2016).
Comparison of serum lipid levels before and after ingesting soy protein snack in 28 hyperlipidemia patients administered a lipid-lowering drug.
Closed bar, before consumption of soy protein snack; open bar, after consumption of soy protein snack.
TG, triglyceride; LDL-C, low-density lipoprotein-cholesterol; HDL-C, high-density lipoprotein-cholesterol; TC, total-cholesterol; Apo-B, apolipoprotein-B.
The results are expressed as means ± SD. * and **: significantly different from placebo group (*p < 0.05 and **p < 0.01).
The author introduces the follow-up result of one of the 28 patients in the above study (Nabiki et al., 2016) in whom triglyceride and total cholesterol levels were reduced following consumption of soy protein snacks (Fig. 4). Even if administration of the statin was stopped, triglyceride level in this patient kept decreasing by eating the soy protein snack. However, after stopping consumption of the soy protein snack and restarting the stain, triglyceride level in this participant once again increased. Upon resumption of consumption of the soy protein snack, triglyceride level decreased to the normal range. In addition, when this participant ceased taking the statin, both triglyceride and cholesterol levels increased despite continuing the soy protein. In another case, there was a participant whose triglyceride level did not change compared to statin alone, despite co-administration of fibrates with statins. However, when ingesting soy protein snacks, the triglyceride level of this participant decreased to a normal level. These results show that lipid-improving drugs and soy protein synergistically normalize triglyceride and cholesterol, and that in case of stopping either the drugs or the soy protein, the effect is weakened. Accordingly, a soy protein snack is recommended for patients with mild dyslipidemia prior to drug therapy or for maladaptive disease patients such as those who have side effects from medications. Because of the required caution needed when combining statin and fibrate, a soy protein snack may be recommended for patients who achieve insufficient results with either drug therapy.

Follow-up result of a soy protein snack and statin on serum lipid levels in dyslipidemic patients (in a single case).
Changes in serum lipid by alternate or simultaneous administration of a statin and soy protein snack.
Closed square, total cholesterol; open square, triglyceride; closed circle, low-density lipoprotein-cholesterol and open circle, high-density lipoprotein-cholesterol.
Soy protein for diabetic nephropathy Metabolic syndrome and nephropathy are closely related; in particular, nephropathy is a complication of diabetes. Moreover, 40% of patients undergoing dialysis are reported to have diabetic nephropathy (Yokoyama et al., 2007). Furthermore, about 50% of type II diabetes patients exhibit urinary albumin disease, which is an early stage of diabetic nephropathy (Parving et al., 2006).
Soy protein has been reported to suppress the progression of diabetic nephropathy (Azadbakht et al., 2008; Asanoma et al., 2012; Jheng et al., 2016). The urine test of diabetic patients in the above-mentioned study (Nabiki et al., 2016) revealed that there were several subjects with high urine albumin levels. These patients have been administered therapeutic agents for treatment of diabetes (sulfonylureas, biguanides, DPP-4 inhibitors and α-glucosidase inhibitors) and dyslipidemia (statins and fibrates). The urinary albumin level decreased by taking soy protein snack, and the improvement on dyslipidemia was also observed (Table 2).
| Before | After | ||
|---|---|---|---|
| PPG | (mg/dL) | 198 | 190 |
| HbA1c | (NGSP%) | 6.6 | 6.5 |
| TG | (mg/dL) | 135 | 100 |
| LDL-C | (mg/dL) | 196 | 189 |
| HDL-C | (mg/dL) | 41 | 34 |
| ALB/Cr | (mg/g Cre) | 94.8 | 24.9 |
Clinical characteristics of subject
Male, age 64, height 161 cm, weight 64 kg (BMI=24.6)
Diagnosis; Type 2 diabetes and diabetic copplication (second stage of nepropathy; eGFR 75.1 mL/min/1.73 m2) during medication for diabetes
PPG, postprandial plasma glucose level; TG, triglyceride, LDL-C, LDL-cholesterol; HDL-C, HDL-cholesterol, ALB/Cre, urinary albumin to creatinine ratio
Soy protein for chronic kidney disease The author introduces another complementary medicine role of soy protein for patients with chronic kidney disease. Combination prescriptions of fibrates and statins for patients with renal dysfunction and dyslipidemia are contraindicated. Thus, in chronic kidney disease, physicians are unable to adequately treat lipid abnormalities.
It has been reported that when chronic kidney disease patients with dyslipidemia ingested β-conglycinin, a major component of soy protein, for 3 months, triglyceride and LDL-cholesterol levels improved without decreasing kidney function. Moreover, renal function of these patients also improved (Ueda et al., 2009, Table 1, protocol 2 and Fig. 5). β-conglycinin has been reported to decrease serum triglyceride levels and visceral fat by inhibiting hepatic fatty acid synthesis (Kohno et al., 2006 and 2012; Ohara et al., 2006; Hori et al., 2009); in addition, β-conglycinin improves insulin sensitivity and lowers triglyceride levels in hypertriglyceridemia (Moriyama et al., 2004; Fukui et al., 2004; Tachibana et al., 2010). Accordingly, β-conglycinin may help to improve lipid abnormalities in patients with renal dysfunction as a complementation medical food material without decreasing kidney function. Moreover, β-conglycinin may improve renal dysfunction as a direct and/or secondary effect of ameliorating lipid abnormalities.
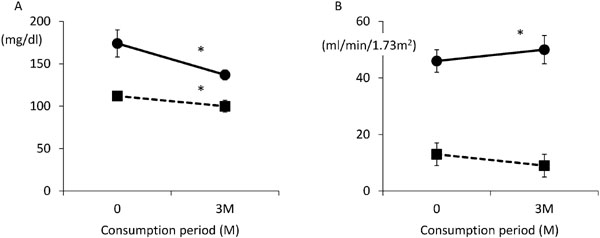
Changes in blood lipid concentrations and renal function among renal function disorder patients with hyperlipidemia (modified from Ueda et al., 2009).
A: Changes for 3 months in triglyceride (TG) and low-density lipoprotein cholesterol (LDL-C).
The black circle shows TG, and the black square shows LDL-C.
B: Changes for 3 months in estimated glomerular filtration rate (eGFR) and blood urea nitrogen/creatinine ratio (BUN/Cre).
The black circle shows eGFR, and the black square shows BUN/Cre.
The results are expressed as means ± SD. *, significant difference between before and after of β-conglycinin consumption (p < 0.05).
In kidney disease, it is recommended that protein intake, including soy, should be carefully monitored and that patients should refrain from excessive protein intake. However, there are several reports showing that mild protein restriction does not suppress the progression of kidney disease (Pan et al., 2008; Koya et al., 2009; Rughooputh et al., 2015). Therefore, it is necessary to consider not only the quantity but also the quality of protein. Soy protein can be regarded as very significant protein to help treat nephropathy.
Background Rheumatoid arthritis is due to inflammation triggered by an immune response to autoantigens. There are 700,000–800,000 patients with rheumatoid arthritis, about 10 million patients with osteoporosis, and close to 700,000–10 million patients with osteoarthritis in Japan (Yoshimura et al., 2009). Many outpatients with rheumatoid arthritis are middle-aged and older women. Many of these patients have swelling and pain due to polyarticular arthritis. Hence, they have difficulties with activities of daily living (ADL), such as cleaning, washing, dressing, and undressing. These patients desire improved ADL through relief of symptoms. Numerous studies have examined the mechanism by which rheumatoid arthritis develops, and new therapeutic agents based on these results have been developed. However, these therapeutic agents have been reported to cause adverse reactions, and some of those reactions are serious (Deane and El-Gabalawy 2014; Kroese et al., 2014). For example, administration of a non-steroidal anti-inflammatory drug can cause a serious adverse reaction such as gastroenteritis or upper gastrointestinal ulceration. Selective COX2 inhibitors are considered to cause fewer adverse reactions, but are reported to cause serious adverse reactions in patients with a history of cardiovascular disease. In addition, inhibiting cytokine action with an antirheumatic drug (biologic or antibody therapy) can lead to various adverse reactions, such as susceptibility to infection, osteoporosis, liver and kidney dysfunction, and myelosuppression. Thus, adequate cautions are warranted when using antirheumatic drugs (Scholes and Smolen 2012).
Soy peptide as an anti-inflammatory agent In elderly patients undergoing rehabilitation after a fall, ingestion of the soy peptide Hinute AM significantly increased muscle mass compared to those who only underwent rehabilitation exercise (Shinkai et al., 2009, Table 1, protocol 3). Interestingly, in this study, in the group who ingested the Hinute AM during rehabilitation exercise, the total white blood cells decreased significantly more than that in the group with only rehabilitation exercise. Total white blood cells increased at the final stage of the inflammatory cascade and is therefore a marker reflecting the inflammatory burden in the body (Pearson et al., 2003). This inflammatory response was suggested to be suppressed by ingesting Hinute AM during rehabilitation exercise. Moreover, an in vitro study using macrophages revealed that Hinute AM inhibited inflammation. In this review, the author introduces the role of Hinute AM as a complementary medicine for the treatment of rheumatoid arthritis (Yoshioka et al., 2015, Table 1, protocol 4).
Soy peptide for rheumatoid arthritis In a double-blind, randomized, parallel-group study for outpatients with rheumatoid arthritis, one group of subjects consumed Hinute AM and the other consumed a placebo. Blood levels of the cartilage matrix degrading enzyme matrix metalloproteinase 3 (MMP-3) and inflammatory cytokines such as IL-1β and IL-6 were measured. The Disease Activity Score 28 (DAS 28, an assessment used by clinicians to measure the disease activity of rheumatoid arthritis) and the Clinical Disease Activity Index (CDAI, an index that measures changes in the disease activity of rheumatoid arthritis) were calculated from the improvement degree of ADL, the severity of pain, and subjective symptoms (visual analog scale, VAS).
The pain score, the physician's assessment of disease activity on a VAS, and the CDAI (which reflects the physician's assessment of disease activity on a VAS) decreased when consuming Hinute AM. The patient VAS score and DAS28-CRP score tended to decrease markedly, and the peptide group had a significantly lower patient VAS score than that in the placebo group. The Hinute AM group had significantly lower levels of IL-6 and IL-1β than those in the placebo group (Fig. 6).
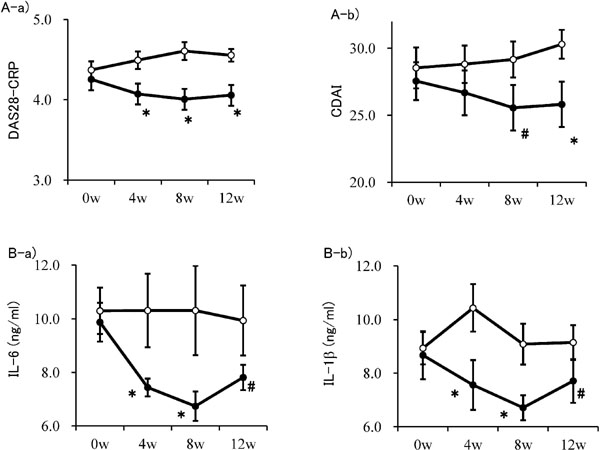
Evaluation of objective and subjective analgesic anti-inflammatory effect (A) and changes of inflammatory marker levels (B) (modified from Yoshioka et al. 2015).
A-a) Disease Activity Score 28-C-reactive protein and A-b) clinical disease activity index. B-a) interleukin-6 and B-b) interleukin-1β.
DAS28-CRP (Disease Activity Score 28-C-reactive protein): Score of degree of rheumatoid arthritis symptoms and inflammation calculated from joint count with pain and swelling for 28 points of articulation, and evaluation of symptoms by the patient (visual analog score [VAS]), and clinical disease activity index (CDAI): Index of activities of daily living improved.
IL, interleukin.
Closed circle: peptide group; open circle: placebo group.
The results are expressed as means ± SD. *: significantly different from placebo group (p < 0.05). #: significantly difference tendency from placebo group (p < 0.1).
IL-6 and IL-1β are closely associated with the pathology of rheumatoid arthritis. The antibody pharmaceutical products (anti-cytokine antibodies) are intended to limit levels of IL-6 and IL-1β in the body (Kaneko and Takeuchi 2014; Gibofsky 2014). IL-6 induces antibody production, causing hypergammaglobulinemia and the emergence of rheumatoid factors (Scholes and Smolen 2012). IL-6 is also a hepatocyte-stimulating factor and increases C-reactive protein and serum amyloid A, which causes secondary amyloidosis (Firestein 2003). In addition, IL-6 activates osteoclasts, induces bone resorption and destruction, and increases joint destruction. Moreover, an increase in blood IL-6 levels is associated with extra-articular symptoms of rheumatoid arthritis such as general malaise, loss of appetite, weight loss, and a slight fever. Consumption of soy peptide decreases IL-6 level.
Subjective assessment by the patient (patient assessment of disease activity on a VAS), physical findings by a physician (physician assessment of disease activity on a VAS and CDAI), and objective assessment (assessment of disease activity on a VAS and the pain score) suggested that patients with rheumatoid arthritis who were being treated with various medications had fewer symptoms of rheumatoid arthritis (pain and arthritis symptoms) as a result of consuming soy peptide. This effect was also evident in a cell experiment using articular chondrocytes from patients with rheumatoid arthritis (Arito et al. 2016). When articular chondrocytes are stimulated with IL-1β, expression of MMP-3, disintegrin, and metalloproteinase with thrombospondin motifs 4 (ADAMTS-4) are activated. MMP-3 activates inflammatory cytokines, ADAMTS-4 is a protease that plays a crucial role in aggrecan cleavage, and ADAMTS-4 is also involved in the degradation of cartilage. Treatment with soy peptide, Hinute AM, significantly suppressed mRNA levels of MMP-3 and ADAMTS-4 enhanced by the IL-1β stimulation. This finding also suggests that Hinute AM may prevent degradation of articular cartilage.
Soy peptide for inflammatory bowel disease Inflammatory bowel disease (IBD) is an intractable disease that causes inflammation of the gastrointestinal tract. Ulcerative colitis and Crohn's disease are the two major pathologies of IBD (Ministry of Health, Labor and Welfare, 2015). Ulcerative colitis is a non-specific inflammatory disorder that causes ulcers and erosion, primarily in the colonic mucosa. An increasing number of patients are certified as having ulcerative colitis, with patients numbering over 170,000 at the end of 2014. Crohn's disease is an inflammatory disorder of unknown etiology that causes chronic granulomatous inflammation that primarily affects different parts of the gastrointestinal tract (from the mouth to the anus) in different patients. The number of patients certified to have Crohn's disease has also increased, reaching over 40,000 at the end of 2014 (Ministry of Health, Labour and Welfare. 2014, Fig. 7).
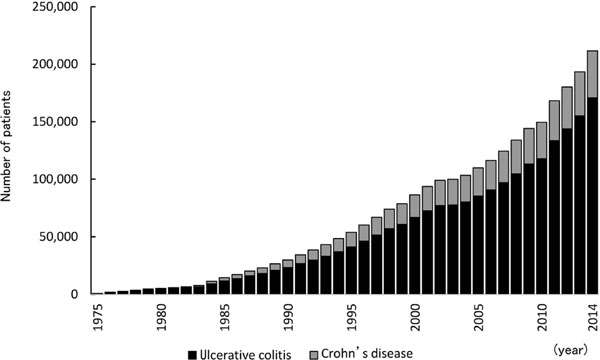
Annual change in the number of patients with Ulcerative colitis and Crohn's disease (drawn from “Number of persons with specific (intractable) disease healthcare certificate” 2015, Japan Intractable Diseases Information Center).
Black bar, number of patients with ulcerative colitis; grey bar, number of patients with Crohn's disease
According to the Japan SPF Swine Society, causes of ulcers in the gastroesophageal region of pigs are defined to be similar to those in humans. Pigs are monogastric and omnivorous animals, so their digestive physiology and intestinal flora are similar to those in humans. The etiology of an enteric infection with a virus or Escherichia coli in neonates is similar to that in piglets, so pigs are a useful model of disease (Miller and Ullrey 1987). The use of dextran sulfate sodium (DSS)-induced colitis has been used as a model of ulcerative colitis (Poritz et al., 2007).
In a study by Young et al. using 5–7-day-old Yorkshire pigs, one group was given a normal saline solution, and two groups were given a normal saline solution containing DSS. Afterwards, the group receiving DSS was given Hinute AM. The other group that was given DSS and the group that was given normal saline were both given a normal saline solution containing alanine to match nitrogen in the soy peptide. The group receiving soy peptide showed almost the same pathology findings compared to that in the group not receiving DSS. Moreover, the expression of genes encoding inflammatory cytokines and protein production of the soy peptide group were also the same compared to that of the no-DSS group (Young et al., 2012). Moreover, Kovacs-Nolan et al. reported that the soy-derived tripeptide Val-Pro-Tyr (VPY) has anti-inflammatory effects in Caco-2 and THP-1 macrophages (in vitro study). They showed that VPY inhibited the secretion of IL-8 and TNF-α in a mouse model of DSS-induced colitis (in vivo study, Kovacs-Nolan et al. 2012). They suggested that tripeptide VPY from soy peptide may be promising for the treatment of IBD.
Soy protein alleviates diminished liver function and diminished kidney function. Soy peptide constituents directly relieve symptoms of rheumatoid arthritis and colitis but do not result in adverse events. In the first half of this review, the author introduced the preventive effect of soy protein on metabolic syndrome, mainly dyslipidemia. This function is thought to be an effect of peptides produced by protein digestion in the body. Research on the identification of beneficial peptide sequences is still in progress. Although soy protein is a superior nutritional and physiological ingredient, further development of soybean into a complementary medical material can be expected by development of the specialized soybean peptide sequence that elicits beneficial physiological function.