2017 Volume 23 Issue 6 Pages 845-853
2017 Volume 23 Issue 6 Pages 845-853
Proteins from taro (Colocasia esculenta (L.) Schott, Betelnut) and sweet potato (Ipomoea batatas (L.) Lam, Tainong No. 57) were hydrolyzed with pepsin and the antioxidative capacities of hydrolysates were analyzed. Hydrolysates of taro or sweet potato were further fractionated into different molecular weight fractions, namely >10 kDa, 5–10 kDa, 1–5 kDa, <1 kDa, and the antioxidative capacity and angiotensin converting enzyme (ACE) inhibitory ability of these fractions were determined. Results indicated peptic hydrolysates from taro and sweet potato proteins exhibited antioxidative capacity and ACE inhibition. High ACE inhibition was found for taro hydrolysate with molecular weight fraction of 1–5 kDa with an IC50 of 0.2571 mg/mL. Antioxidative capacity of molecular weight fraction of taro decreased with decreasing molecular weight. Molecular weight fraction of > 10 kDa from sweet potato was found to have better antioxidative capacity. Regardless species, molecular weight fraction with >10 kDa had the highest antioxidative capacities.
The biological activity of protein hydrolysate mainly depends on several factors, including (1) the type of enzyme used, (2) hydrolysis conditions, and (3) composition, sequence and peptide structure of amino acid (Tavano, 2013). The features of protein hydrolysates, such as solubility, cohesion, foaming ability, and emulsify capacity will be altered after proteolysis. Nalinanon et al. (2011) pointed out in their research that Alcalase and Flavourzyme hydrolysis of Nemipterus virgatus muscle had rendered decreased emulsion stability and foamability while increased solubility of the hydrolysates. Moreover, the solubility of hydrolyzed peanut protein was 80% higher than peanut protein extract (Jamdar et al., 2010). Peanut protein and whey protein can suppress the activity of angiotensin I converting enzyme after trypsin hydrolysis (Vermeirssen et al., 2002). In addition, storage protein in Ipomoea batatas (L.) Lam displayed elevated antioxidant activity after pepsin hydrolysis (Huang et al., 2007b).
The area under cultivation of Colocasia esculenta (L.) Schott in Taiwan is extremely extensive, among which, Colocasia esculenta var. esculenta (Betelnut), Colocasia esculenta var. esculenta (Kaohsiung No.1) and Colocasia esculenta var. esculenta (Men-Yu) are the common representative varieties. Moreover, Colocasia esculenta (L.) Schott is also extensively cultivated in Japan, which is associated with plenty of varieties (Hirai et al., 1989). Water content in fresh Colocasia esculenta (L.) Schott is about 63.6%–72.4%, while starch content is 21.1%–26.2%, and protein content is 1.75%–2.57%; besides, it also contains a large amount of potassium and magnesium (Huang et al., 2007a). The leaf and stem of Colocasia esculenta (L.) Schott was reported to protect the liver from oxidative injury and prevented the diabetic complications (Aniya et al., 2002). In addition, the edible tubers of Colocasia esculenta (L.) Schott possessed the abilities of antioxidation, anti-tumor and anti-bacterial growth (Wei et al., 2011). Extract of Colocasia esculenta (L.) Schott leaf showed anti-hypertensive and diuretic effects (Otari et al., 2012).
The edible roots of Ipomoea batatas (L.) Lam have different colors which can be attributed to the diverse contents of β-carotene, anthocyanin and minerals (Grabowski et al., 2008; Woolfe, 1992). In addition to protein and niacin, Ipomoea batatas (L.) Lam also contains abundant vitamin A precursor, β-carotene, the content of which is even higher than that in carrot. Ylönen et al. (2003) suggested that carotenoids contributed to stabilizing the plasma glucose concentration and improving insulin resistance. Meanwhile, vitamins C and E can protect cell content (Bouwkamp, 1985). Additionally, Ipomoea batatas (L.) Lam displayed anti-cancer and anti-oxidative effects (Teow et al., 2007; Tian and Wang, 2008), while methanol extract of Ipomoea batatas (L.) Lam could also suppress mouse melanoma B16-induced liver injury (Hayase and Kato, 1984; Shimozona et al., 1996; Suda et al., 1997).
Angiotensin I-converting enzyme (ACE, EC. 3.4 15.1) is zinc-containing exo-metalloprotease, which exists in vascular endothelial cell, epithelial cell and neuroepithelial cell, with the most abundance in lung (Fang et al., 2008). Angiotensin converting enzyme inhibitor (ACEI) is a competitive inhibitory substance which has greater affinity to ACE than angiotensin I and bradykinin. Furthermore, it is unlikely to be released from ACE active region and therefore can effectively achieve the inhibitory effect (Wei et al., 1992). Natural food protein-derived inhibitory peptides are safe and have side effects. Therefore, numerous researches have demonstrated proteins or peptides with ACE inhibitory activity from natural foods. Jauhiainen and Korpela (2007) suggested that consumption of foods containing antihypertensive peptides could effectively contributed to reducing the blood pressure. Therefore, proteins in Colocasia esculenta (L.) Schott and Ipomoea batatas (L.) Lam were hydrolyzed using pepsin in this study for antioxidative capacity and ACEI analyses. Moreover, the hydrolysates were fractionated according to different molecular weights with an aim to obtain peptides possessing antioxidant and ACE inhibitory activity.
Taro and sweet potato protein extraction Colocasia esculenta (L.) Schott variety (Betelnut taro) and the Ipomoea batatas (L.) Lam (Tainong No. 57) sweet potato were purchased from the local supermarket. Protein extraction was conducted according to the procedure of Chen and Lin, 2007) with modification. Taro and sweet potato were washed, peeled and cut into 1–2 cm cubes and blended with 50 mM Tris-HCl buffer (pH=8.3) at a ratio of 1:4 (w/v) in the homogenizer (Waring Blendor, Model 34BL97, Dynamics Corporation of America, New Hartford, Conn., U.S.A.). Extraction was carried out for 4 h at 4°C with gentle stirring, followed centrifugation for 30 min at 12,500 × g (High-Speed Centrifuge, Avanti J-25, Beckman Coulter Inc., Palo Alto, Calif., U.S.A.). The supernatant was precipitated with 100% ammonium sulfate at 4°C, followed by centrifugation for 15 min at 12,500 × g. The precipitate was dissolved in tenfold volumes (w/v) of 50 mM Tris-HCl buffer (pH=8.3) and dialyzed overnight to remove ammonium sulfate. Crude protein extract of the Colocasia esculenta (L.) Schott and Ipomoea batatas (L.) Lam were obtained.
Protein concentration determination Protein concentration was determined using bicinchoninic acid (BCA) assay (Hill and Straka, 1988). Bovine serum albumin (BSA) or samples were diluted with deionized water to concentrations of 0.1–1 mg/mL. One milliliter of standard working solution was added and mixed with 20 µL sample. The absorbance was determined at the wavelength of 562 nm using the UV/Vis Spectrophotometer (Beckman, DU530).
Pepsin hydrolysis The pH values of Colocasia esculenta (L.) Schott and Ipomoea batatas (L.) Lam crude protein extracts were adjusted to 2.0 using 2 N HCl followed by the addition of pepsin (Sigma, ≥250 units/mg) at an enzyme substrate ratio of 1:1 (w/w). Hydrolysis was carried out at 37°C for 8 h followed by heating at 90°C for 10 min for inactivation of enzyme. The mixtures were cooled immediately in an ice-bath and the pH of supernatant was adjusted to 7.0, and the hydrolysate was obtained for subsequent analysis.
Protein hydrolysate fractionation The molecular weight fractionation was conducted as proposed by He et al. (2013) with modification. Protein hydrolysates with various molecular weights were separated using the tangential flow filtration (Labscale TFF system, Millipore Corporation, Billerica, MA, U.S.A.). Protein hydrolysates with the molecular weights of >10 kDa, 5–10 kDa and <5 kDa were collected by the 10 kDa (Biomax 10) and 5 kDa (Biomax 5) molecular weight cut-off membranes, respectively. Moreover, protein hydrolysates with the molecular weight of 1–5 kDa and <1 kDa were separated with the Stirred Cell apparatus (Millipore) using the Ultracel l kDa Ultrafiltration Disc (regenerated cellulose, 1,000 NMWL, Millipore). The unfractionated mixture and all sample fractions (>10 kDa, 5–10 kDa, 1–5 kDa and <1 kDa) were freeze dried using Freeze Dryer FD-5N (Eyela, Tokyo Rikakikai CO., LTD. Koishikawa Bunkyo-ku, Tokyo, Japan) and stored at −20°C for further analysis.
DPPH(1,1-diphenyl-2-picrylhydrazyl) radical scavenging capacity Four hundreds microliters of Tris-HCl buffer and 500 µL 100 mM DPPH ethanol solution were added to 100 µL sample solution (various concentrations of protein hydrolysates with different molecular weights) according to the method of Yamaguchi et al. (1998). The mixed solutions were allowed to react for 20 min in a dark place, and the absorbance was measured at 517 nm. In addition, the effective concentration at 50% inhibition (EC50) was calculated.
Superoxide anion scavenging capacity Three hundreds microliters of 0.1 M phosphate buffer (pH 7.4), 80 µM phenazine methosulfate (PMS), 624 µM NADH, and 200 µM nitroblue tetrazolium (NBT) were added to 300 µL sample solution of varying concentrations based on the method by Gao et al. (1998). The absorbance of the mixed solutions at 560 nm was determined. A lower absorbance suggested stronger superoxide anion scavenging ability of the sample. The scavenging effect (%) of superoxide anion was calculated as the following formula: [1-(A560 after adding the extract/A560 in the control group with no addition of extract)]× 100%.
Ferrous ion chelating capacity A 3.7 mL methanol aliquot was added to 1 mL sample solution of various concentrations according to the method by Dinis et al. (1994). Following the addition of 0.1 mL of 2 mM FeCl2 and 0.2 mL of 5 mM ferrozine, the absorbance at 562 nm was taken 10 min. A lower absorbance indicates higher ability of chelating ferrous ion of the sample.
Reducing power Three hundreds microliters of phosphate buffer and 1% K3Fe(CN)6 were mixed with 0.3 mL solution fractionated with various protein molecular weights for the determination of reducing power (Oyaizu, 1986). The mixture was mixed evenly and heated at 50°C in a water bath for 20 min. The mixture was then cooled in ice-bath and mixed with 0.3 mL of 10% TCA. The mixture was centrifuged at 6000 × g for 10 min. A 0.6 mL aliquot of the supernatant was mixed with 0.6 mL deionized water and 0.12 mL of 0.1% FeCl3, and the absorbance was determined at 700 nm. A higher absorbance represents stronger reducing power of the solution fraction.
ACE inhibition activity N-[3-(2-Furyl)acryloyl]-L-phenylalanyl-glycyl-glycine (FAPGG) was used as the reaction substrate of ACE according to the methods by Vermeirssen et al. (2002) and Huang et al. (2006a). A 50 M Tris-HCl buffer (containing 0.3 M NaCl, pH=8.4) solution was utilized to prepare 1 mM FAPGG solution. A 200 µL sample aliquot was mixed with 1 mL of 1 mM FAPGG solution, followed by heating in water bath for 5 min at 37°C. Following the addition of 20 µL ACE, the absorbance was measured using the time scan manner (1 reading/10 sec) for 10 min at 345 nm. ACE inhibitory activity (%) was calculated according to the formula: [(1-(ΔA inhibitor) ÷ ΔA control)] × 100%, and the Inhibition Concentration at 50% (IC50) was calculated. FAPGG at the concentrations of 0.4, 1 and 5 mM was treated as the substrate to determine the inhibition pattern of protein hydrolysate fractions on ACE activity. Moreover, changes in absorbance at different were used to calculate the reaction rate V (ΔA/min). Meanwhile, Vmax and Km were calculated using the Lineweaver-Burk plot.
The protein hydrolysates of taro (Colocasia esculenta (L.) Schott, Betelnut) and sweet potato (Ipomoea batatas (L.) Lam, Tanong No. 57) with various molecular weights possessed the DPPH free radical scavenging capacity (Fig. 1). Additionally, each molecular weight fraction was dose-dependent, with DPPH scavenging rate being increased with the increase in tested concentration. Regardless of species, protein hydrolysates of unfractionated mixture had the highest DPPH free radical scavenging capacity. Taro protein hydrolysate with molecular weight >10 kDa had the best DPPH free radical scavenging capacity among four hydrolysate fractions. The EC50 was calculated to be 11.92 mg/mL according to the linear regression equation. The hydrolysate with the molecular weight of 5–10 kDa ranked the second in terms of DPPH free radical scavenging capacity, while that of <1 kDa was the lowest in DPPH free radical scavenging capacity. The DPPH free radical scavenging capacity of sweet potato protein hydrolysates showed similar trend with that of taro protein hydrolysates. The concentrated solution had the best DPPH free radical scavenging capacity with an EC50 of 7.44 mg/mL. Meanwhile, the protein hydrolysate with the molecular weight of >10 kDa had the best DPPH scavenging capacity, with an EC50 of 12.17 mg/mL, while that of <1 kDa had the lowest capacity. Results indicated that Taro protein hydrolysates that had not been separated with membrane possessed the highest DPPH free radical scavenging capacity (EC50 of 6.68 mg/mL). At the same molecular weight of individual fraction, taro protein hydrolysates had higher DPPH free radical scavenging capacity than that of sweet potato.

DPPH scavenging capacity of different protein hydrolysate fractions from taro and sweet potato.
The superoxide anion scavenging capacities of protein hydrolysates (mixed and fractionated) of taro (Colocasia esculenta (L.) Schott, Betelnut) and sweet potato (Ipomoea batatas (L.) Lam, Tainong No. 57) were shown in Fig. 2. It could be seen that regardless of cultivars the unfractionated, concentrated protein hydrolysate had the highest superoxide anion scavenging capacity, with taro being the highest with an EC50 of 6.68 mg/mL. The molecular weight of >10 kDa among taro protein hydrolysate fractions had the best superoxide anion scavenging capacity (EC50=7.24 mg/mL), while that of <1 kDa had the lowest superoxide anion scavenging capacity. Such trend was similar to that of DPPH free radical scavenging capacity. In general, taro protein hydrolysates had higher superoxide anion scavenging capacities than those of sweet potato, with the best effect being seen in concentrated solution (EC50 of 9.81 mg/mL). At the same time, the fractionated protein hydrolysate with the molecular weight of >10 kDa had the best superoxide anion scavenging capacity (with the EC50 of 10.95 mg/mL), which was similar to the taro protein hydrolysate fraction with molecular weight of <1 kDa. The protein hydrolysate fraction of sweet potato with molecular weight of <10 kDa had low capacity (lower than 10%).

Superoxide anion scavenging capacity of different protein hydrolysate fractions from taro and sweet potato.
Taro protein hydrolysate of with molecular weight of 5–10 kDa or above possessed the ability of chelating ferrous ion (Fig. 3), with the highest ability being found in the concentrated solution (EC50 of 8.00 mg/mL), while those of 1–5 kDa and <1 kDa only had ferrous ion chelating ability of 10%. In contrast, different sweet potato protein hydrolysate fractions had low ferrous ion chelating abilities; among which, 1–5 kDa and <1 kDa fractions appeared to have no ferrous ion chelating abilities.

Ferrous ion chelating capacity of different protein hydrolysate fractions from taro and sweet potato.
The reducing power of different protein hydrolysates of taro and sweet potato was shown in Fig. 4. It was observed that the overall trend in reducing power was similar to that of ferrous ion chelating ability, which showed a dose-dependent effect. The concentrated solution (not isolated with membrane) and molecular weight of >10 kDa of taro protein hydrolysates had the highest absorbance of 2.257 and 2.177, respectively, suggesting the highest reducing abilities. The absorbance of concentrated solution and molecular weight of >10 kDa of sweet potato protein hydrolysates also appeared to be the highest (2.000 and 1.926, respectively), while molecular weight of 1–5 kDa and <1 kDa had the lowest reducing power.

Reducing ability of different protein hydrolysate fractions from taro and sweet potato.
Figures 5 and 6 shows the ACE inhibitory activities of protein hydrolysates of taro (Colocasia esculenta (L.) Schott, Betelnut) and sweet potato (Ipomoea batatas (L.) Lam, Tainong No. 57). The taro protein hydrolysate fractions with molecular weight of 1–5 kDa and <1 kDa had higher inhibitory activities, with an EC50 value of 0.38 mg/mL and 0.26 mg/mL, respectively; while protein hydrolysate fraction with the molecular weight of >10 kDa had the lowest inhibitory activity. The ACE inhibitory activities of sweet potato protein hydrolysates were lower than those of taro (Fig. 6). The highest ACE inhibitory ability was noticed in sweet potato protein hydrolysate with the molecular weight of 1–5 kDa, with an IC50 value of approximately 0.396 mg/mL, followed by the molecular weight of <1 kDa (IC50 1.98 mg/mL). The protein hydrolysates with molecular weight of 5–10 kDa and >10 kDa had low ACE inhibitory activities.

ACE inhibition capacity of different protein hydrolysate fractions from taro.
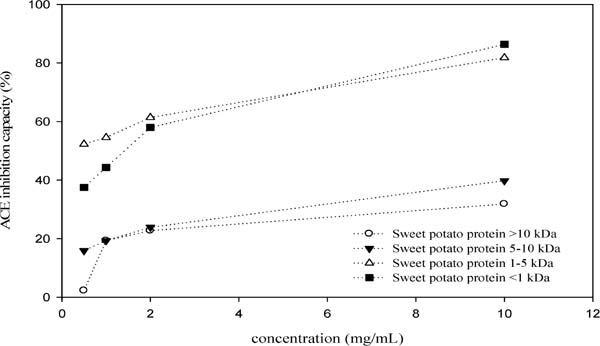
ACE inhibition capacity of different protein hydrolysate fractions from sweet potato.
Regardless of species, taro tuber protein hydrolysate with molecular weight of 1–5 kDa had the best ACE inhibitory activity among all protein hydrolysate fractions. Therefore, the ACE activity inhibition enzyme dynamics, and its inhibitory mode was analyzed using the Lineweaver-Burk plot. Under different concentrations (0, 0.1, 0.2, 0.3 mg/mL) of protein hydrolysates, FAPGG at varying concentrations was hydrolyzed by ACE and plotted against enzyme velocity (ΔA/min) (Fig.7). The Vmax and Km of the control (with no protein hydrolysate) was 0.0576 and 1.28 mg/mL, respectively. In contrast, the apparent Km of various concentrations of taro protein hydrolysates with molecular weight of 1–5 kDa was 1.589, 2.973 and 4.62 mg/mL, respectively, but Vmax remained almost unchanged. Thus, it was obvious that the ACE inhibitory mode of taro protein hydrolysate with molecular weight of 1–5 kDa was competitive inhibition.

Competitive inhibition of molecular weight fraction 1–5 kDa from taro using various FAPGG concentrations.
The DPPH free radical scavenging capacities of taro and sweet potato protein hydrolysates were dose-dependent. Current results were consistent with previous results by Huang et al. (2006b) and Santos and Gonçalves (2016). Taro protein hydrolysates with large molecular weight had higher scavenging capacity, which displayed the same trend with the research by Vieira et al. (2017). Vieira et al. (2017) reported fractionation of Brewers' spent grain proteins into <10 kDa and <3 kDa and found molecular weight of <10 kDa exhibited ferric ion reducing antioxidant power (FRAP) in addition to protecting Caco-2 and HepG2 cell lines from being attacked by free radicals, which was dose-dependent. The authors also suggested that such protective ability may derive from its antioxidant capacity. However, the fraction of <3 kDa has reduced antioxidant power; therefore, tests on its effects on protecting Caco-2 and HepG2 cell lines had not been conducted. Sweet potato protein hydrolysate with molecular weight >10 kDa had the best scavenging rate as well. Hou et al. (2005) pointed out that hydrolysates obtained from sporamin (storage protein in Ipomoea batatas (L.) Lam) hydrolyzed by pepsin and/or chymotrypsin had superb antioxidant property. Furthermore, Hou et al. (2001) and Huang et al. (2007b) also suggested that sporamin showed excellent antioxidant capacity. In this study, the unfractionated protein hydrolysates had the highest antioxidant capacities (DPPH free radical scavenging capacity, superoxide free radical scavenging capacity and ferrous ion chelating capacity), regardless of cultivars. This may be attributed to the storage proteins remained in the mixed solution, which was in accordance with previous results.
In addition, Terahara et al. (2004) suggested that the antioxidant power of sweet potato (Ipomoea batatas (L.) Lam) was associated with anthocyanin. Meanwhile, taro and sweet potato used in this study contained anthocyanins (Hsiao, 2011; Simsek and Nehir El 2015; Tseng, 2010) which were too large to pass through the molecular weight cut-off membrane and were thus remained in the mixed concentrated solution and large molecular weight fraction. Haiwei (2010) suggested that proteins with small molecular weight have high superoxide free radical scavenging capacity and those with the molecular weight of <1 kDa had the best effect on scavenging superoxide free radical. However, in this research, the unfractionated protein hydrolysates and those of large molecular weight (>10 kDa) of taro and sweet potato had the best effect which was due to the trypsin inhibitors (TIs), with respective molecular weight of 24 kDa and 28 kDa, that possessed superoxide anion free radical scavenging capacity (Hammer et al., 1989; Hou et al., 2005). Besides, different protein hydrolysates and fractions hydrolyzed by different enzyme systems resulted in different effects on superoxide anion scavenging capacity (He et al., 2013). Thus, the disagreement might result from different protein hydrolases and fractions.
Results in this research suggested that protein hydrolysates of both taro and sweet potato with molecular weight over 5 kDa have high ferrous ion chelating activity, while those with molecular weight of <5 kDa exhibited poor activity. This result agreed with that of rapeseed protein hydrolysates reported by He et al. (2013). In addition, increasing the length of peptide chain could synergistically increase ferrous ion chelating activity with amino acid residues in comparison with short chain peptide (Tang et al., 2009). Furthermore, the hydrophobic force on the surface of protein molecule may be also related to ferrous ion chelation.
Reducing ability determination is mainly employed to evaluate whether the antioxidants can reduce the ferric ion into ferrous ion (Duh and Yen, 1997), and the antioxidants can provide electron to reduce the ferricyanide into ferrous state (Wojdylo et al., 2007). Taro protein hydrolysates have higher reducing ability than sweet potato protein hydrolysates. This accounts for the reason that taro protein hydrolysates have stronger ability to produce electrons than sweet potato protein hydrolysates. Taro protein hydrolysates with molecular weight of >1 kDa are the major electron donors, while sweet potato protein hydrolysates with the molecular weight of >5 kDa are the dominant electron providers. Zhu et al. (2008) and Borawska et al. (2016) suggested that the released free amino acids were served as the additional electrons and protons to maintain reduction at the time of hydrolysis. Additionally, amino acid side groups with various reducing capacities would also be exposed, which manifested the ability to expose the electron density region, thus showing different reducing capacities.
Taro and sweet potato protein hydrolysates showed inhibition on ACE activity (Otari et al., 2012; Ishiguro et al., 2012). In this research, peptides with ACE inhibitory activity is obtained through rapid screening of acetone extract of rabbit lung (Vermeirssen et al., 2002). The results suggested that among pepsin hydrolysates of water soluble protein in taro, those with molecular weight of < 10 kDa possess strong ACE inhibitory capacity. Among them, the molecular weight of 1–5 kDa had the best ACE inhibitory capacity. No difference in inhibitory activity was noted between <1 kDa and 1–5 kDa; nonetheless, 1–5 kDa manifested the highest ACE inhibitory activity at the lowest tested concentration (0.1 mg/mL). This demonstrated that pepsin hydrolysates of taro (Colocasia esculenta (L.) Schott, Betelnut) with the molecular weight of 1–5 kDa have high inhibitory activity. Sweet potato protein hydrolysates with molecular weight of <1 kDa showed the highest inhibitory activity at high tested concentration (10 mg/mL). Our results agreed with those reported by Huang et al. (2011a, 2011b) who concluded that amino acid residues of 7–8 revealed ACE inhibition acidity. On the other hand, protein hydrolysate of 1–5 kDa fraction had the highest inhibitory activity at the lowest tested concentration (0.5 mg/mL) indicating higher ACE inhibitory activity.
Taro (Colocasia esculenta (L.) Schott, Betelnut) tuber and sweet potato (Ipomoea batatas (L.) Lam) root are the traditional staple foods in Taiwan, Japan, Southeast Asian, and many other countries in the world. However, few studies on the antioxidant activity and ACE inhibitory activity focus on the water soluble proteins in taro tuber and sweet potato root. Results in this research have demonstrated the molecular weights of proteins possessing antioxidant activity and ACE inhibitory activity in taro tuber and sweet potato root after pepsin digestion. This seems to be beneficial to consumer health who frequently consumer these foods. Nonetheless, the molecular weight of these hydrolysates to be absorbed by the small intestine may require further research. However, based on current and other research results there is a potential application on these peptides into a health food, food ingredient, or other types of foods. Subsequent research on separation, purification and sequencing the bioactive peptide fragments are recommended.
ACKNOWLEDGES This research was funded in part by the National Science Council (Project No. NSC 98-2313-B-126-007-MY3), Executive Yuan, Taiwan and appreciation is acknowledged.