2018 Volume 24 Issue 1 Pages 129-137
2018 Volume 24 Issue 1 Pages 129-137
Our previous study indicated that certain commercially available proteases inhibit biofilm formation by cariogenic streptococci. Natto made from soybeans cultured with Bacillus subtilis natto contains an abundance of proteolytic enzymes. In this study, we investigated the correlation between the protease activity of extracts from 36 commercially available natto products and the inhibition of biofilm formation. The biofilm inhibitory effect was found to correlate with the level of protease activity in the natto extracts, without reducing the viable cell numbers. The natto extract markedly inhibited the production of a water-insoluble glucan by Streptococcus mutans, which is the main agent involved in the formation of biofilm by cariogenic streptococci. The characteristics of the protease present in the extract were similar to those of nattokinase. Our results indicate that the protease activity exhibited by extracts of the Japanese fermented food natto reduces the risk of caries by inhibiting biofilm formation.
The gram-positive oral bacteria Streptococcus mutans and Streptococcus sobrinus are the principal etiologic agents of dental caries in humans (Kuramitsu et al., 2007). The pathogenic potential of these species is attributed to their high ability to produce acids, their high degree of acid tolerance, the presence of high-affinity systems for the assimilation of numerous carbohydrate sources, and their biofilm-forming abilities. Therefore, it is thought that the relative numbers of these bacteria in dental biofilms is linked to incidence of dental caries. In both S. mutans and S. sobrinus, the synthesis of a water-insoluble glucan from sucrose promotes adhesion to the tooth surface and the aggregation of bacterial cells within biofilms (Narisawa et al., 2011). In S. mutans, water-insoluble glucan is synthesized by glucosyltransferases, which are encoded by gtfB and gtfC. The inactivation of these genes has been shown to result in the dramatic loss of the ability to form biofilms (Narisawa et al., 2011). Numerous additional factors such as aggregation by salivary agglutinins (Ahn et al., 2008), the presence of extracellular DNA as a biofilm matrix (Petersen et al., 2005), and the development of quorum sensing (Tamura et al., 2009; Wang and Kuramitsu, 2005) are involved in biofilm formation.
Proteases are protein-hydrolysing enzymes that are present in all living organisms, in which they display many essential physiological functions. Proteases are a diverse group of enzymes, differing in structure, target substrate, reaction mechanism and many physicochemical properties (Hedstrom, 2002). Recent reports have shown that proteases to bacteria in industrial, environmental, and healthcare settings have often interfered with biofilm formation and/or destroyed mature biofilm (Mukherji et al., 2015; Thallinge et al., 2013). Conversely, bacterial extracellular protease functions to cleave the host protein and contributes to tissue damage and dissemination (Connolly et al., 2011). To reduce the risk of the development of caries, safe anti-adherence agents are needed.
Natto, a traditional Japanese non-salt-fermented food product made from soybeans, has been popular for more than 1,000 years and is currently one of the most widely consumed fermented foods in Japan. There are three main types of natto: non-salted natto (Itohiki-natto), salted natto (Hama-natto), and cracked natto (Yukiwari-natto). Among these, Itohiki-natto is the most popular and is produced in largest amounts. Henceforth, the term “natto” will be used specifically to describe Itohiki-natto in this paper. The manufacture of natto generally involves the following four steps: soaking, steaming or boiling, inoculation of a starter culture, and incubation. A detailed description of each process has been provided in previous reports (Kiuchi and Watanabe, 1989; Murooka and Yamshita, 2008). Fermented products have high nutritional value, characteristic flavour, and high viscosity, and are covered with a white mucilaginous substance (Kiuchi and Watanabe, 1989; Sugawara et al., 1985). The metabolic characteristics of Bacillus subtilis as a starter culture and the fermentation conditions used also affect the quality of the fermented products obtained (Kubo et al., 2011; Schallmey et al., 2004; Wei et al., 2001). It is known that proteases secreted by starter cultures are very active and effectively degrade soy proteins into oligopeptides and amino acids.
In our previous study, naturally occurring proteolytic agents were found to interfere with sucrose-dependent biofilm formation by S. mutans and S. sobrinus, without reducing the bacterial growth rate (Narisawa et al., 2014). Although the detailed inhibitory mechanism of these enzymes was not elucidated, we considered that the enzymes might inhibit the growth of S. mutans by interfering with sucrose-dependent biofilm formation. However, there have been no reports on the effectiveness of natto, which contains large amounts of these proteolytic enzymes, against biofilm formation by cariogenic streptococci. The objective of this study was to investigate the putative ability of commercially available natto to inhibit biofilm formation by cariogenic streptococci.
Bacterial strains and culture conditions The bacterial strains used in this study, S. mutans UA159 (genome strain), S. mutans GS5, S. mutans FSM-11 (clinical isolate) (Narisawa et al., 2011), S. sobrinus AHT, S. gordonii DL1, S. gordonii ATCC 10558, S. sanguinis ATCC 10556, S. salivarius HT9R, and S. salivarius ATCC 9759, were maintained in brain-heart infusion medium (BHI) (Becton Dickinson and Company, San Jose, CA) and cultured at 37°C in an atmosphere containing 5% CO2.
Preparation of natto and soybean extracts Thirty-six commercially available fermented soybean products, which were within their expiration dates, were selected. Extracts were prepared as follows: 40 g of natto was homogenised in 360 mL of distilled water in a BagMixer stomacher laboratory blender (Interscience, St. Nom, France) for 3 min. When required, the extracts were filtered using an Aspirator filter (0.2 µm; Millipore, Bedford, MA). Soybean extracts were prepared similarly to the natto extracts by using soybeans soaked in distilled water for 16 h at room temperature.
Viable cell count, crude protein, and saccharide content of extracts The crude protein content of cell-free natto extracts and soybean extracts was determined using a protein assay kit (Thermo Fisher Scientific, Kanagawa, Japan) with bovine serum albumin as a standard. The viable cell numbers were determined as colony-forming units (CFU) using appropriate decimal dilutions of the extracts in sterile distilled water, which were plated on BHI agar and incubated at 37°C for 24 h. The glucose, fructose, and sucrose content in the extracts was measured using a Saccharose/d-Glucose/d-Fructose F-kit (JK International, Tokyo).
Protease activity Proteolytic activity was measured based on the hydrolysis of azo-casein (Narisawa et al. 2014). One unit of proteolytic activity was defined as the amount of the enzyme required to produce an increase of 0.001 absorbance units at 440 nm per minute at pH 7.4.
Biofilm formation assays
Microtitre plates Aliquots (2 µL) of cultures and 80 µL of 2 × tryptic soy broth (TSB) without dextrose (Becton Dickinson and Company), supplemented with 0.5% sucrose, were added to 96- well polystyrene microtitre plates (Sumitomo Bakelite, Tokyo) and then cell-free natto extract and/or sterile distilled water were added to a total volume of 200 µL. Before the addition of a cell suspension, the wells were coated with an artificial saliva Saliveht® aerosol (Teijin Pharma, Tokyo). The plates were incubated at 37°C in an atmosphere of 5% CO2 for 20 h. After the incubation, the plates were washed with distilled water, and adherent cells were stained with safranin. The dye was solubilized in 70% ethanol, and the optical density of at 492 nm was determined using a microplate reader (Colona Electric, Ibaraki, Japan).
Flow cells Flow cells (Stovall Life Science, Greensboro, NC) with the artificial saliva were inoculated with S. mutans UA159. After 3-h incubation at 37°C without a flow, the medium with or without 50% (v/v) cell-free natto extract was added at a rate of 3.0 mL·h−1 for 16 h using a peristaltic pump. To investigate the dispersion of biofilm, the medium with 50% (v/v) cell-free natto extract was added to the cells after 16 h of cultivation and then the culture was allowed to continue for additional 16 h under the above conditions. After cultivation the flow cells were washed twice with phosphate-buffered saline (PBS).
Microscopic analysis Aliquots (100 µL) of an S. mutans UA159 culture and 15 mL of 2 × TSB without dextrose, supplemented with 0.5% sucrose, were added to a 50-mL tube, and then cell-free natto extract and/or sterile distilled water were added for a total volume of 30 mL. An artificial saliva-coated glass slide was placed horizontally in the medium. The tubes were incubated at 37°C and 5% CO2 for 20 h. After the glass slide was washed with PBS, the biofilm was directly stained on the glass with 4′,6-diamidino-2-phenylindole dihydrochloride n-hydrate (DAPI) (Wako, Osaka, Japan) or Alexa Fluor 594-conjugated concanavalin A (Thermo Fisher Scientific). The staining solutions were prepared as described previously (Kensche et al., 2013). Samples were observed under a BX60 fluorescence microscope (Olympus, Tokyo). Each sample was recorded at five randomly selected positions.
Growth studies
Viable cell numbers Culture tubes were prepared as described above and incubated at 37°C with 5% CO2 for 20 h. Aliquots (50 µL) of cultures and 2.5 mL of 2 × TSB without dextrose, supplemented with 0.5% glucose, were added to culture tubes, followed by cell-free natto extract and/or sterile distilled water to a total volume of 5.0 mL. The viable cell count of S. mutans was evaluated as CFU using appropriate decimal dilutions of the culture in sterile distilled water, which were plated on BHI agar and incubated at 37°C for 2 days.
Inhibitory assay on an agar plate To evaluate the inhibitory effects of natto extracts against streptococci, 5 µL of an overnight culture was spotted on a BHI agar plate. A cell-free natto extract (5 µL) was spotted near enough to the colony to almost touch it. The plate was incubated at 37°C with 5% CO2 for 2 days.
Quantification of water-insoluble glucan derived from cariogenic streptococci The water-insoluble glucan content was estimated using the phenol–sulphuric acid method (Narisawa et al., 2014). Briefly, adherent cells in the micro-tube were centrifuged at 18,000 g for 10 min. The precipitate was dissolved in 1.0 M NaOH. The alkali-soluble polysaccharide was precipitated using ethanol. The precipitate was washed, dried, and resuspended, and its volume was estimated using glucose as a standard.
Zymography Extract for zymography was prepared as follows: 40 g of natto was homogenised in 80 mL of PBS in a BagMixer stomacher laboratory blender (Interscience) for 3 min. The extracts were precipitated with 20% (w/v) ammonium sulphate at 4°C for more than 2 h. After centrifugation, the supernatant was further precipitated with 80% (w/v) ammonium sulphate. The supernatants obtained after centrifugation were dialysed against 100 mM PBS (pH 7.0). The samples were analysed using a casein– polyacrylamide gel (15% acrylamide), which was prepared by mixing with 0.1% (w/v) casein. A 5-µg sample was loaded onto the gel and electrophoresed. Thereafter, the gel was washed with distilled water containing 2.5% Triton X-100 and incubated for 1 h at 37°C with 50 mM Tris–HCl buffer (pH 7.4) containing 0.05% CaCl2 (w/v) and 0.85% NaCl (w/v). When required, leupeptin (Wako), phenylmethylsulfonyl fluoride (PMSF) (Sigma–Aldrich, St. Louis, MO), or tosyl-L-phenylalanyl-chloromethane (TPCK) (Sigma–Aldrich) was added to the buffer to a final concentration of 1.0 mmol·L−1 as a protease inhibitor. The gel was stained with Coomassie brilliant blue R-250 (Wako).
Statistical analysis All data are expressed as the mean ± SD. Data were statistically analysed using Student's t-test. Values of P < 0.05 were considered to be statistically significant.
Thirty-six commercially available natto products were used for the preparation of natto extracts. The proteolytic activities of the 36 cell-free natto extracts ranged from approximately 0.44 ± 0.08 to 1.84 ± 0.12 U (Table 1). These results agreed with a previous report in which the protease activities of commercially available natto products were shown to differ greatly (Akimoto et al., 1990). We also investigated the pH value, crude protein content, viable cell number, and saccharide content of each natto extract. Variations in pH were observed; the pH of the samples was within the range of 6.1 to 7.6. The number of viable cells in the natto extracts ranged from 6.36 ± 0.32 log CFU/mL to 9.49 ± 0.17 log CFU/mL. The colonies obtained from individual samples showed typical single and B. subtilis-like morphology. The crude protein content ranged from 0.64 ± 0.11 mg/mL to 5.81 ± 0.30 mg/mL. The glucose content ranged from 3.03 ± 0.62 to 289.15 ± 25.00 µg/mL in all samples, with the exceptions of samples No. 1, 9, 10, 11, 12, 14, 16, 21, and 32, which had no detectable glucose content. Fructose was detected in all samples, whereas sucrose was hardly detected in any sample. As shown in Fig. 1, the biofilm formation decreased with the increase in protease activity in the natto extract (S. mutans UA159, R2 = 0.82; S. mutans FSM-11, R2 = 0.81; S. sobrinus AHT, R2 = 0.86). The values of pH, viable cell numbers, amounts of crude protein, and saccharide levels (glucose, fructose, and sucrose) in the natto extracts (Table 1) did not affect the biofilm formation (R2 < 0.01). The inhibitory effect of a natto extract on biofilm formation was also confirmed in flow cell systems (Fig. 2a, b). Berg et al. (2001) described that proteases from Euphausia superba efficiently released microorganisms from a plaque. In the present study, bacterial biofilm could not be detached upon perfusion of the broth with a cell-free natto extract after 16 h of cultivation (Fig. 2c, d).
| No. | Origin | pH | Viable cell numbers (Log CFU/ml) | Crude protein (mg/ml) | Glucose (µg/M) | Fructose (µg/M) | Sucrose (µg/M) | Protease activity (U) |
|---|---|---|---|---|---|---|---|---|
| 1 | manufacture natto | 7.6 | 8.23±0.10 | 4.36±1.04 | N.D.† | 80.52±1.00 | N.D. | 1.84±0.12 |
| 2 | 7.6 | 8.74±0.30 | 3.10±0.09 | 20.16±1.00 | 81.10±5.58 | N.D. | 0.81±0.12 | |
| 3 | 7.3 | 8.45±0.07 | 4.30±0.38 | 20.74±3.46 | 148.88±5.58 | N.D. | 1.49±0.02 | |
| 4 | 7.2 | 8.61±0.04 | 2.67±0.01 | 10.36±3.0 | 53.88±0.00 | N.D. | 1.20±0.18 | |
| 5 | 7.2 | 8.64±0.12 | 5.06±0.33 | 16.12±1.00 | 214.94±2.64 | 5.48±3.78 | 1.07±0.22 | |
| 6 | 7.2 | 8.36±0.24 | 5.13±0.09 | 5.76±0.20 | 78.78±2.64 | 6.56±3.28 | 1.09±0.02 | |
| 7 | 7.3 | 8.91±0.03 | 3.07±0.08 | 26.66±3.00 | 402.06±4.38 | N.D. | 0.67±0.58 | |
| 8 | 7.6 | 8.11±0.29 | 5.81±0.30 | 16.74±3.58 | 258.5±13.34 | N.D. | 0.84±0.04 | |
| 9 | 7.6 | 8.03±0.21 | 3.53±0.14 | N.D. | 94.30±24.88 | N.D. | 1.25±0.02 | |
| 10 | 7.5 | 9.49±0.17 | 0.78±0.06 | N.D. | 32.44±2.64 | N.D. | 1.52±0.04 | |
| 11 | 6.8 | 6.36±0.32 | 4.06±0.24 | N.D. | 31.29±8.61 | 18.6±6.64 | 0.55±0.05 | |
| 12 | 7.2 | 9.08±0.08 | 2.52±0.75 | N.D. | 128.18±10.44 | 9.30±0.95 | 1.20±0.07 | |
| 13 | 7.2 | 8.07±0.12 | 3.40±0.23 | 3.03±0.62 | 115.65±22.02 | 19.69±0.00 | 1.04±0.04 | |
| 14 | 6.1 | 9.28±0.02 | 1.45±0.15 | N.D. | 42.15±9.21 | 13.95±1.16 | 0.72±0.04 | |
| 15 | 6.9 | 7.77±0.06 | 3.72±0.11 | 8.64±2.45 | 234.92±8.53 | 33.64±1.16 | 1.03±0.05 | |
| 16 | 7.0 | 8.16±0.05 | 3.17±0.39 | N.D. | 132.09±10.68 | N.D. | 1.53±0.02 | |
| 17 | 6.6 | 7.86±0.11 | 3.98±0.47 | 9.51±7.33 | 284.75±13.19 | 30.31±3.55 | 1.95±0.06 | |
| 18 | 6.6 | 8.18±0.09 | 2.85±0.03 | 289.15±25.00 | 125.40±15.67 | N.D. | 1.31±0.05 | |
| 19 | 7.3 | 8.63±0.07 | 4.22±0.69 | 4.61±0.99 | 80.39±3.72 | N.D. | 1.59±0.10 | |
| 20 | 7.0 | 7.89±0.06 | 4.27±0.28 | 9.50±2.99 | 101.24±11.67 | 16.41±2.32 | 1.00±0.14 | |
| 21 | 7.3 | 7.99±0.05 | 5.37±0.21 | N.D. | 143.06±15.21 | 22.15±3.48 | 1.48±0.04 | |
| 22 | 7.1 | 7.76±0.07 | 3.28±0.65 | 6.63±2.78 | 81.43±0.37 | N.D. | 1.77±0.06 | |
| 23 | 7.0 | 7.72±0.05 | 3.97±0.52 | 159.56±11.80 | 74.15±7.89 | N.D. | 0.81±0.06 | |
| 24 | 7.1 | 7.18±0.16 | 3.04±0.24 | 6.63±1.00 | 38.81±5.09 | 9.85±0.00 | 1.08±0.04 | |
| 25 | 7.3 | 8.43±0.04 | 3.10±0.45 | 6.91±0.00 | 73.86±3.13 | 7.39±1.17 | 1.39±0.06 | |
| 26 | 7.2 | 8.05±0.09 | 2.17±0.12 | 16.42±3.77 | 99.50±9.22 | N.D. | 0.44±0.08 | |
| 27 | 7.1 | 8.36±0.03 | 3.02±0.35 | 21.03±8.65 | 132.67±4.79 | N.D. | 1.40±0.07 | |
| 28 | 7.1 | 7.86±0.08 | 3.22±0.14 | 5.18±0.00 | 56.92±4.30 | N.D. | 0.93±0.09 | |
| 29 | 7.2 | 8.27±0.03 | 3.03±0.28 | 8.21±1.83 | 28.68±7.38 | N.D. | 1.29±0.21 | |
| 30 | 7.2 | 8.10±0.06 | 4.71±0.08 | 16.42±1.73 | 18.69±4.31 | N.D. | 1.76±0.20 | |
| 31 | 7.0 | 7.47±0.16 | 1.73±0.42 | 7.78±1.73 | 78.50±4.11 | N.D. | 1.35±0.21 | |
| 32 | 7.5 | 8.14±0.16 | 3.71±0.17 | N.D. | 29.55±3.68 | 11.49±3.28 | 1.16±0.04 | |
| 33 | 7.0 | 8.31±0.09 | 2.56±0.11 | 7.78±4.89 | 56.20±3.29 | N.D. | 1.45±0.02 | |
| 34 | 6.8 | 8.13±0.21 | 2.64±0.12 | 9.21±4.76 | 157.29±6.14 | 28.99±6.63 | 0.92±0.04 | |
| 35 | 7.1 | 7.97±0.13 | 0.64±0.11 | 28.23±5.75 | 97.62±10.62 | 5.75±1.17 | 1.05±0.09 | |
| 36 | 7.1 | 8.10±0.03 | 5.34±0.24 | 27.93±4.43 | 38.59±2.78 | 24.77±2.64 | 1.65±0.12 | |
| 37 | soy beans | 7.1 | N.D. | 2.59±0.05 | 36.00±2.64 | 37.93±1.36 | 902.56±127.64 | N.D. |
| 38 | 7.1 | N.D. | 2.06±0.08 | 27.65±1.73 | 34.76±0.00 | 1202.86±39.45 | N.D. | |
| 39 | 7.1 | N.D. | 2.68±0.34 | 25.92±1.73 | 27.23±5.92 | 701.53±24.37 | N.D. | |
| 40 | 6.9 | N.D. | 2.89±0.62 | 37.24±1.42 | 43.74±2.19 | 685.12±107.91 | N.D. |

Effects of natto and soybean extracts on biofilm formation by cariogenic streptococci: (a) Streptococcus mutans UA159; (b) S. mutans FSM-11; (c) S. sobrinus AHT. Each extract (50%, v/v) was added to the medium. The white circles represent natto extracts, and the grey circles represent soybean extracts. Regression curves were obtained based on the data for natto extracts. Data represent average values from three independent experiments. Dotted lines indicate the biofilm volume in the control.

Effects of natto extract No. 21 on biofilm formation by Streptococcus mutans UA159 in flow cells. The panels show the results of biofilm formation after (a) 16 h of cultivation without the natto extract; (b) 16 h of cultivation with the natto extract; (c) 32 h of cultivation without the natto extract; and (d) 16 h of cultivation without the natto extract, followed by 16 h of cultivation with the natto extract. The data are representative of three independent experiments.
We also investigated the effect on biofilm formation of using soybeans as a raw material for the natto production. We found that the extracts of four types of soybeans promoted the formation of biofilm by cariogenic streptococci, as evidenced by the increase in biofilm biomass (Fig. 1, grey circles).
Treatments with three randomly selected cell-free natto extracts did not significantly reduce the number of viable cells of S. mutans UA159 (Fig. 3A), and one of the cell-free natto extracts (sample No. 21) had no effect on the growth of eight oral streptococci (Fig. 3B). A fibrinolytic enzyme from Bacillus licheniformis shows strong lytic activity against S. mutans (Kim et al., 1999), but we did not observe any lytic activity against S. mutans in the natto extracts (data not shown). None of the cell-free soybean extracts significantly promoted the viable cell numbers of S. mutans (Fig. 3A). Natto extracts No. 21, 32, and 34 lost their proteolytic activity and promoted biofilm formation when treated at a temperature of 80°C (Fig. 4). A previous study has shown that plant-based pectin promoted the formation of biofilm by S. mutans (Kawarai et al., 2016). In general, the primary role of pectin in biofilm formation is due to its binding to cell–cell and/or cell–carrier gaps. Natto contains approximately 37% soluble nitrogen-free products, which are composed of oligosaccharides derived from the cell walls of soybeans (Taguchi et al., 1986). S. mutans may promote biofilm formation by using the oligosaccharides derived from soybeans.

Effects of natto and soybean extracts on the viability of cariogenic streptococci. (a) Each extract (50%, v/v) was added to the growth medium. Data represent average values from three independent experiments (*P < 0.05). There were no significant differences (P > 0.05). (b) The dotted circles represent spots of cell-free natto extract No. 21.
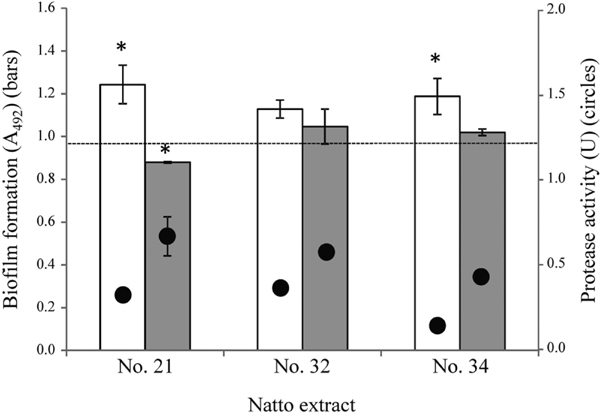
Effects of heat treatment (white bars) and protease inhibitor PMSF (gray bars) on natto extract No. 21, No.32, and No.34. Heat treatment of the medium with 50% (vol/vol) natto extract was performed for 15 min at 80°C. PMSF was added to the medium with natto extract (50%, vol/vol) at a final concentration of 1.0 mmol·L−1. Dotted lines indicate the biofilm volume in the control. The biofilm formation is representative of three experiments (vs. control, *P < 0.05). Non-labelled pairs in the biofilm data are not significantly different from each other (vs. control, P > 0.05). The protease activities are representative of three independent experiments (circles).
Previous studies have shown that an N-acetylmuramidase from Streptomyces globisporus, named mutanolysin (E.C. 3.2.1.17), had proteolytic activity and inhibited the biofilm formation by interfering directly with glucosyltransferase, which synthesises the water-insoluble glucan from sucrose (Nara and Morioka, 1985). After 20-h cultivation of S. mutans UA159 without natto extract, the water-insoluble glucan content in the cultures was 100.11 ± 8.06 µg·mL−1, whereas those in the cultures grown in the presence of 50% (v/v) natto extract samples No. 21, 32, and 34 were 41.96 ± 8.21, 50.92 ± 6.59, and 77.11 ± 6.46 µg·mL−1, respectively. These results indicate that a biofilm inhibition factor(s) present in natto extracts influences the glucosyltransferase activity. We found significant differences in the water-insoluble glucan content between cultures grown with or without natto extracts (P < 0.05). Glucan formation and bacterial cells were visualised by simultaneous staining with concanavalin A and DAPI (Fig. 5). S. mutans appeared to form compact and island-like aggregates (Fig. 5a, d). The S. mutans culture incubated with natto extract No. 21 showed visibly fewer bacteria and smaller microcolonies (Fig. 5b, e). The microscopic results were in agreement with those observed using microtitre plates and flow-cell systems. In a control experiment, the adherent cells were covered by large amounts of a material, which was suggested to consist of exopolysaccharides (Fig. 5g). The amount of exopolysaccharides appeared to decrease in the presence of the extract (Fig. 5h). The component saccharides in S. mutans biofilm are mainly water insoluble glucans, and they are synthesized using GtfB- and GtfC-glucosyltransferases from sucrose (Narisawa et al., 2011). The potential anti - glucosyltransferase of the proteolytic enzyme of natto should be investigated in future experiments.

Microscopic analysis of biofilm formation by cells of Streptococcus mutans UA159 on a glass slide. (a, d, and g) Control cells were grown in the growth medium. (b, e, and h) Natto extract No. 21 (50%, v/v) was added to the growth medium. (c, f, and i) Natto extract No. 21 (50%, v/v) and PMSF (final concentration of 1.0 mmol·L−1) were added to the growth medium. a–c, Phase contrast images; d–f, DAPI-stained images; g–i, concanavalin A-stained images. Bars = 10 µm.
To characterise the proteases present in natto extracts, a concentrated natto extract from commercially available natto sample No. 21 was assayed by zymography. The fractions from the purification steps were combined to yield an overall purification of 4.61-fold (Table 2). As shown in Fig. 6, the main protease present in the extract was approximately 27 kDa (Fig. 6, lane 1). The band could not be detected at a final PMSF concentration of 1.0 mmol·L−1 (Fig. 6, lane 2) but was detected in the presence of leupeptin and TPCK (Fig. 6, lanes 3 and 4, respectively).
| Purification | Total activity (U) | Total protein (mg) | Specific activity (U/mg) | Purification (fold) | Biofilm inhibition (%)* |
|---|---|---|---|---|---|
| Crude supernatant | 3.71 | 19.01 | 0.21 | 1 | 73.75 |
| 20% (NH4)2SO4 precipitation | 2.16 | 9.34 | 0.24 | 1.14 | 81.86 |
| 80% (NH4)2SO4 precipitation | 6.47 | 6.64 | 0.97 | 4.61 | 93.91 |

Zymography of the natto extract protease. Concentrated samples from natto extracts No. 21 were applied to casein gels. (a) Control sample; (b) PMSF-treated sample; (c) leupeptin-treated sample; (d) TPCK-treated sample; (e) marker.
In the presence of 1.0 mmol·L−1 PMSF, the proteolytic activity in natto extract samples No. 21, 32, and 34 decreased to 43.92%, 48.28%, and 44.57% of the original activity, respectively. The inhibitory activity of the extracts on the formation of biofilm by S. mutans UA159 was also abolished in the presence of the same concentration of PMSF (Fig. 4, and Fig. 5c, f, i). The presence of PMSF did not significantly reduce the growth of S. mutans UA159 (data not shown).
The standard laboratory strain B. subtilis 168 is not suitable for use as a natto starter culture, as this strain possesses at least 8 genes that encode extracellular proteases (http://natto-genome.org/gene. html). Among these, subtilisin, which is encoded by aprE and commonly referred to as the major exoprotease, accounts for the majority of the total extracellular protease activity of B. subtilis (Dabbagh et al., 2014). Nattokinase is structurally similar to subtilisin and is produced by the natto starter strain B. subtilis natto, and it is abundantly present in natto products and contributes to characteristics such as the taste and quality of the end product (Kada et al., 2013). Nattokinase is a single-chain structure comprising 275 amino acid residues with a molecular weight of 27.7 kDa, and has no intramolecular disulphide bonds (Zheng et al., 2005). Previous results have indicated that PMSF at a final concentration of 100 µmol·L−1, but not TPCK or leupeptin, completely inhibited the activity of a purified nattokinase (Fujita et al., 1993). We previously found that commercially available nattokinase strongly inhibits biofilm formation without impacting the components of the culture medium (Narisawa et al., 2014). These results support our speculation that at least one of the proteases present in the natto extracts was nattokinase.
S. mutans possesses a variety of protein factors that enable it to survive and persist in the oral niche. Glucan-binding protein is one such extracellular protein and is involved in aggregation and biofilm formation (Banas et al., 1997; Biswas et al., 2007; Sato et al., 2002). Recent in vivo studies reported that glucan-binding protein contributes to the cariogenicity of S. mutans through a mechanism that involves the alteration of biofilm architecture (Lynch et al., 2013). Additional studies utilizing a purified biofilm-related protein factor from S. mutans as well as glucosyltransferase are needed to test the anti-biofilm effects of natto in proteolytic enzymes.
To our knowledge, this study is the first to reveal the potential of natto to reduce the risk of caries. We will evaluate the effect of the agents under conditions more similar to an actual environment, such as the development of multi-species biofilms and the presence of salivary amylase, in addition to their safety for the host. Semi-purified enzymatic extracts from natto extract in this study did not achieve the complete inhibition of biofilm or the destruction of mature biofilm. In the future, proteolytic enzymes from natto might be applicable as an anticaries agent in combination with other enzymes, such as mutanase and dextranase (Johansen et al., 1997).
In this study, we found that natto extracts inhibited sucrose-dependent biofilm formation by cariogenic S. mutans without affecting the growth rate of the bacterium. Our previous findings indicated that proteolytic enzymes strongly inhibit cariogenic biofilm formation. The effects of natto extract on S. mutans observed in this study correlated with the protease activity. The characteristics of the protease present in the extract were similar to those of nattokinase. The composition of fermented natto products is highly varied and complex; the target factors involved in biofilm inhibition may be identified via the use of inhibitors, as reported in the present study. The purification of the protease from natto may be useful for developing oral care products such as toothpaste. Further investigation is required to elucidate the mechanisms by which this enzyme exerts anti-biofilm effects.
Acknowledgements This work was supported in part by the Takano Life Science Research Foundation and by Grants-in-Aid for the Development of Scientific Research (No. 15K18704) from the Ministry of Education, Science and Culture of Japan.