2018 Volume 24 Issue 1 Pages 169-175
2018 Volume 24 Issue 1 Pages 169-175
Pomegranate juice (PJ) has higher polyphenol content and antioxidative activity than any other fruit juice. We used a pomegranate polyphenol concentrate (PPC) from pomegranate juice and investigated its inhibitory effects and mechanism of action using a contact hypersensitivity (CHS) test. The ear swelling induced by 2,4-dinitrofluorobenzene (DNFB) was inhibited in the PPC-treated group compared to that in the control group. Antigen-specific IgG1 was lower in the PPC-treated group than in the control group; however, serum antigen-specific IgG2a was not different. More splenic IL-10-producing and IFN-γ-/IL-4-producing CD4+ T cells were observed in the PPC-treated group than in the control group. These results suggested that PPC afforded protection against CHS and IgG1 production by increasing IL-10-producing CD4+ T cell numbers.
Allergic contact dermatitis (ACD) is one of the most common skin diseases that are caused by delayed-type hypersensitivity responses to antigens that come into contact with the skin (Kaplan et al., 2012). Small organic molecules that are chemically reactive with proteins form the most important category of contact allergens. They bind to self-proteins to generate immunogenic antigens through a process termed haptenization (Meglio et al., 2011). Contact allergens are common in cosmetics, personal care products, and jewelry. Examples of common allergens are organic chemicals such as those found in fragrances and dyes, and inorganic chemicals such as nickel (Peiser et al., 2012).
Contact hypersensitivity (CHS) is an experimental animal model for human ACD (Honda et al., 2013). CHS is characterized by T-cell mediated antigen-specific skin inflammation induced by topical skin contact with haptens in previously sensitized hosts. The CHS response develops in two phases: an afferent phase and an efficient phase. The afferent phase is induced by initial skin contact with the hapten. Skin dendritic cells capture the hapten and subsequently migrate to the draining lymph nodes where they prime specific T cells. These T cells differentiate into CHS effectors in 5–7 days, which recirculate through the blood. The efficient phase develops after the second skin contact to the same hapten and leads to a skin inflammatory response that peaks 24–48 h after a challenge (Inagaki and Nagai, 2009; Vocanson et al., 2009; Martin et al., 2011).
Pomegranate (Punica granatum L.) fruits are widely consumed fresh and as beverages. Pomegranate juice (PJ) contains a higher total polyphenol content compared to that in other fruit juices such as orange, grape, grapefruit, and apple juice. The content of soluble polyphenols in PJ varies between 0.2%–1.0% (Aviram and Rosenblat, 2012). This high content of polyphenols, particularly ellagitannins (ETs), has been associated with the health effects of PJ, which include potential preventive effects against chronic diseases such as cancer, diabetes, and cardiovascular diseases (Ghosh and Scheepens, 2009; Faria and Conceicao, 2011).
ETs belong to the chemical class of hydrolysable tannins and pomegranate contains ETs such as punicalagin and punicalin (Landete, 2011). The bioavailability of ETs from PJ has been studied in human subjects. The ETs were hydrolyzed to yield ellagic acid (EA), which was further metabolized in the gastrointestinal tract by the colon microflora to chiefly urolithin derivatives (mainly urolithin A, UA) (Cerdá et al., 2004; Seeram et al., 2006). ETs, EA, and UA have been demonstrated to possess various biological properties, including antioxidant, anticancer, anti-atherosclerotic and anti-inflammatory activities in in vitro and in vivo studies (Heber, 2008; Larrosa, 2010). Therefore, a number of biological properties of pomegranate are believed to be related to ETs and their derivatives (Ito et al., 2014).
To the best of our knowledge, however, the effects of pomegranate polyphenols in CHS have not been investigated. In the present study, we used a pomegranate polyphenol concentrate (PPC) from pomegranate juice and investigated its inhibitory effects and mechanisms of action on CHS.
Pomegranate polyphenol concentrate A commercial pomegranate aril extract (from Iran), which was chromatographed over Diaion HP-20 (Sigma-Aldrich, St. Louis, MO, USA) to remove sugar (Kawakami et al., 2014), was used as PPC in the present study. The obtained PPC contained 59.5% phenolics, which were determined by the Folin-Denis method using ethyl gallate as a standard. The detailed polyphenolic compounds in PPC were reported previously (Kawakami et al., 2014; Ito et al., 2014).
Animals Female BALB/c mice (6 weeks old, 15–20 g body weight) were purchased from Japan SLC (Hamamatsu, Japan). The mice were housed in a room at 25 ± 2°C with a 12-h light/dark cycle, and provided acidified water and MF diet (Oriental Yeast, Tokyo, Japan) ad libitum. The experimental design was in accordance with the guidelines for animal experimentation and was approved by the Animal Experimental Committee of Kawasaki University of Medical Welfare (authorization number 12-003) and Kawasaki Medical School (authorization number 12-036).
Contact hypersensitivity test The mice were divided into two groups, which were provided acidified water (control group, n = 6) or water supplemented with 0.2% PPC (PPC-treated group, n = 6), respectively. The mice were sensitized on day 0 by applying 100 µL 0.5% 2,4-dinitrofluorobenzene (DNFB, Kanto Chemical, Tokyo, Japan) diluted in acetone/olive oil (4:1) on the shaved dorsal skin. In the 0.5% DNFB challenge experiment, the mice were challenged by the application of 20 µL 0.5% DNFB on both sides of the ears on day 7. In the 0.15% DNFB challenge experiment, the mice were challenged by application of 20 µL 0.15% DNFB on both sides of the ears on days 7 and 14. The ear thickness was measured using a soft touch micrometer (CLM1-15 QM, Mitsutoyo, Tokyo, Japan). All animal experimental procedures were performed under sevoflurane anesthesia (Maruishi Pharmaceutical, Osaka, Japan).
IgG1- and IgG2a-specific ELISA Blood was obtained from the orbital veins of the mice while under sevoflurane anesthesia on days 0 and 8 for the 0.5% DNFB challenge, and on days 0, 8 and 15 for the 0.15% DNFB challenge. Obtained sera were analyzed using an enzyme-linked immunosorbent assay (ELISA) specific for IgG1 and IgG2a against dinitrophenol-labeled human serum albumin (DNP-HSA, Sigma-Aldrich). Flat-bottomed microtiter plates were precoated with 100 µL DNP-HSA (10 µg/mL) in 0.1 M carbonate buffer (pH 9.6), and were incubated overnight at 4°C. After the wells had been washed with PBS containing 0.05% Tween 20 (PBST), 1% BSA/PBST solution was added to each well, and the plates were incubated for 1 h at 37°C. A volume of 100 µL of each serum sample diluted 50 × and 30 × for the measurement of IgG1 and IgG2a levels, respectively, with 1% BSA/PBST was subsequently added to each well, followed by incubation for 1 h at 37°C. After washing each well with PBST, 100 µL of anti-mouse IgG1-HRP (diluted 1:1000 in 1% BSA/PBST; Southern Biotechnology, Birmingham, AL, USA) or anti-mouse IgG2a-HRP (diluted 1:1000 in 1% BSA/PBST; Southern Biotechnology) was added to each well, and the solution was incubated for 1 h at 37°C. Each well was washed with PBST, and 100 µL of o-phenylenediamine (0.4 mg/mL) in a citrate buffer (pH 5.0) containing 0.006% H2O2 was added. The reaction was quenched with 50 µL of 2 N H2SO4 (aq.) after 5 min at room temperature, and color development was measured by colorimetric photometry at 492 nm.
Intracellular cytokine staining The mice in the 0.15% DNFB challenge experiment were sacrificed under anesthesia on day 20 of the experiment. The spleen was immediately excised and splenic single cells were suspended in RPMI-1640 medium (Sigma-Aldrich) containing 10% (v/v) fetal calf serum, 100 U/mL penicillin, and 100 µg/mL streptomycin (Sigma-Aldrich). The cells (1 × 107 cells/well) were stimulated in 6-well plates for 5 h with 50 ng/mL phorbol 12-myristate 13-acetate (PMA; Sigma-Aldrich) and 500 ng/mL ionomycin (Sigma-Aldrich) in the presence of protein transport inhibitor (containing monensin, BD Bioscience, Bedford, MA, USA) at 37°C under a humidified atmosphere containing 5% CO2. The stimulated cells were fixed with BD fixation buffer (BD Bioscience), permeabilized with BD perm/wash buffer (BD Bioscience), and stained with fluorescence-labeled antibodies against the respective intracellular cytokine and CD4 expressed on the cell surface, phycoerythrin- (PE) conjugated rat anti-mouse interferon-gamma (IFN-γ, clone: XMG1.2), PE-conjugated rat anti-mouse interleukin- (IL) 4 (clone: 11B11), PE-conjugated rat anti-mouse IL-10 (clone: XMG1.2), PE-conjugated rat IgG1 κ isotype control (clone: R3-34), and fluorescein isothiocyanate- (FITC) conjugated rat anti-mouse CD4 (clone: RM4-5) antibodies (BD Bioscience) according to the manufacturers' instructions. The stained cells were analyzed using a BD FACSCalibur™ (BD Bioscience).
Statistical analysis The data represent the mean ± SD. The statistical significance of two groups was analyzed by a Student's t-test using the Origin 8.5 software (OriginLab, Northampton, MA, USA). The data were considered significantly different at p < 0.05.
Suppressive effects of PPC on DNFB-induced ear swelling in mice In the 0.5% DNFB challenge experiment, the sensitized mice were challenged with 0.5% DNFB on day 7, and the ear thickness was measured as a function of time (Fig. 1A). The ear swelling induced by 0.5% DNFB was significantly inhibited in the PPC-treated group compared to that in the control group. In the 0.15% DNFB challenge experiment, the sensitized mice were challenged with 0.15% DNFB on days 7 and 14, and the ear thickness was measured as a function of time (Fig. 1B). The ear swelling was significantly inhibited in the PPC-treated group compared to that in the control group on day 8 (24 h after the first challenge) and on day 16 (48 h after the second challenge). Commonly, a CHS response is induced by application of DNFB to the ear of DNFB-sensitized mice. The degree of ear swelling correlates with the intensity of the effector response and the swelling peaks 24–48 h after the challenge (Honda et al., 2013). Therefore, these results indicated that CHS responses were suppressed by oral feeding of PPC.
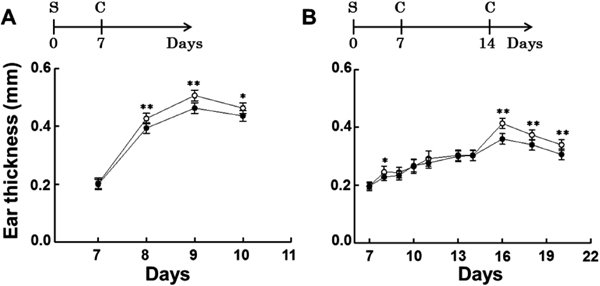
Feeding mice with PPC attenuates ear swelling due to contact allergy. BALB/c mice were fed either 0.2% pomegranate polyphenol concentrate (PPC, PPC-treated group, ●) or acidified water (control group, ◯). Mice in each group were sensitized (S) with 0.5% 2,4-dinitrofluorobenzene (DNFB), and were challenged (C) with 0.5% or 0.15% DNFB as described in the experimental protocols. Ear thickness was measured using a micrometer. A) 0.5% DNFB challenging experiment. B) 0.15% DNFB challenging experiment. Each value represents the mean ± SD of groups of six mice each; *p < 0.05, **p < 0.01 vs. the control.
Inhibition of hapten-specific IgG responses in mice by oral feeding of PPC Figure 2A and B show DNP-specific IgG1 and IgG2a levels, respectively, on days 0 and 8 in the 0.5% DNFB challenge experiment. The DNP-specific IgG1 and IgG2a levels were low and not significantly different between the PPC-treated and control groups.

The effect of feeding mice with PPC on hapten-specific IgG1 and IgG2a levels in the serum of DNFB-challenged mice. Pomegranate polyphenol concentrate- (PPC) treated or control- (acidified water) treated mice were sensitized with 0.5% 2,4-dinitrofluorobenzene (DNFB), and were challenged with 0.5% or 0.15% DNFB as described in the figure legend of Fig. 1. Serum samples were obtained at the indicated days and dinitrophenol- (DNP) specific IgG1 and IgG2a levels were measured by ELISA. A) IgG1 and B) IgG2a levels in 0.5% DNFB-challenged mice. C) IgG1 and D) IgG2a levels in 0.15% DNFB-challenged mice. Each value represents the mean ± SD of groups of six mice each; **p < 0.01 vs. the control.
Figure 2C and D show DNP-specific IgG1 and IgG2a levels, respectively, on days 0, 8, and 15 in the 0.15% DNFB challenge experiment. The DNP-specific IgG1 and IgG2a levels were similarly low as those measured in the 0.5% DNFB challenge experiment on day 8. The serum IgG1 level in the PPC-treated group measured on day 15 was higher than that in the control group, while the IgG2a level on day 15 was not significantly different between the PPC-treated and control groups.
Repeated DNFB-applications induce DNP-specific IgG1 (Th2-type) and IgG2a (Th1-type) in mice (Chapat et al., 2004). The present study indicates that oral feeding of PPC inhibits hapten-specific IgG1 production, but not IgG2a production, induced by skin sensitization with DNFB.
Effect of PPC feeding on the activation of splenic cells CD4+ and CD8+ T cells can be subdivided into Th1/Tc1 and Th2/Tc2 subsets. IFN-γ- and IL-4-producing CD4+ cells are indicators of Th1 and Th2 cells, respectively. On the other hand, IFN-γ- and IL-4-producing CD8+ cells are markers of Tc1 and Tc2 cells, respectively (Honda et al., 2013). In a preliminary experiment, we examined IFN-γ-producing CD4+ and CD8+ cells that were obtained from the spleens of PPC-treated and control groups in the 0.15% DNFB challenge experiment. The number of IFN-γ-producing CD4+ cells in the PPC-treated group was higher than that in the control group, but the number of IFN-γ-producing CD8+ cells was not different between the PPC-treated and control groups (data not shown). Thus, in the subsequent experiments, we studied CD4+ cells that produced IFN-γ, IL-4, or IL-10. Figure 3 shows FACS profiles for the splenocytes of the PPC-treated and control groups, indicating the intracellular expression of the respective cytokine (IFN-γ, IL-4, or IL-10) and CD4 expression on the cell surface.
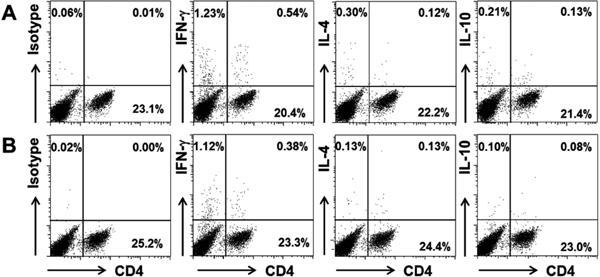
FACS profiles of splenocytes obtained from DNFB-challenged mice. BALB/c mouse splenocytes were stimulated with phorbol 12-myristate 13-acetate (PMA) and ionomycin in the presence of monensin for 5 h. The cells were fixed, permeabilized, and stained for the respective intracellular cytokine (interferon-gamma [IFN-γ], interleukin- [IL] 4, or IL-10) and cell surface-expressed CD4. The stained cells were analyzed by FACS. A) Pomegranate polyphenol concentrate- (PPC) treated mice group. B) Control- (acidified water) treated mice group.
The number of IFN-γ-producing cells was not different between the PPC-treated and control groups, but that of the IFN-γ-producing CD4+ cells was significantly increased in the PPC-treated group compared to that in the control group. In contrast, the numbers of both IL-4-producing cells and IL-4-producing CD4+ cells were not different between the PPC-treated and control groups (Fig. 4). These results indicate that the number of IFN-γ-producing CD4+ cells (Th1) was increased, while that of IL-4-producing CD4+ cells (Th2) was not altered in the PPC-treated group compared to that in the control group. In other words, the Th1/Th2 balance might be altered toward the Th1-dominant state by oral feeding of PPC. This could be a reason for the observation that oral feeding of PPC inhibited hapten-specific serum IgG1 production but not IgG2a production (Fig. 2C). Gao et al. (2005) established an allergic dermatitis model in NC/Nga mice by repeated exposure of the mice to a mite antigen, and demonstrated that the oral administration of four Kampo medicines, i.e., Juzen-taiho-to, Hochu-ekki-to, Shofu-san, and Oren-gedoku-to, inhibited mite antigen-induced ear swelling and the elevation of the serum IgE level. In addition, the Kampo medicines prevented not only the increase in IL-4 mRNA expression but also the decrease in IFN-γ mRNA expression in the cervical lymph nodes, indicating that the Kampo medicines altered the Th1/Th2 balance toward the Th1- dominant state. The authors concluded that the Kampo medicines might contribute to the inhibition of dermatitis by altering the Th1/ Th2 balance. Furthermore, these Kampo medicines contain polyphenols that also might contribute to the inhibition of dermatitis.

The effect of feeding mice with PPC on activated mouse splenocytes. Splenocytes were obtained from 0.15% 2,4-dinitrofluorobenzene- (DNFB) challenged and polyphenol concentrate- (PPC) treated or control- (acidified water) treated mice. The cells were stimulated and stained for the respective intracellular cytokine (interferon-gamma [IFN-γ], interleukin- [IL] 4, or IL-10) and cell surface-expressed CD4 as described in the figure legend of Fig 3. The stained cells were analyzed by FACS. A) Cytokine-producing cells as the percentage of the total number of cells. B) Cytokine-producing CD4+ cells as the percentage of the total number of CD4+ cells. Each value represents the mean ± SD of six independent experiments; *p < 0.05, **p < 0.01 vs. the control.
In the present study, the auricular application of 0.15% DNFB was repeated twice and DNP-specific IgE was not detected in the sera of mice. Gao et al. (2004) reported that DNP-specific serum IgE was detected after more than five auricular applications of 0.15% DNFB. This could be a reason why serum IgE was not detected in our animal model. Furthermore, in our previous study, we demonstrated that ET metabolites such as urolithin A inhibited IgE-mediated allergic responses in an in vitro model system. The metabolites highly suppressed antigen-induced degranulation and secretion of allergy-related cytokines (IL-4 and TNF-α) in RBL-2H3 cells (Nagano et al., 2012). These results suggest that PPC is a potential therapeutic method for treating not only ACD but also atopic dermatitis.
The number of IL-10-producing cells was not different between the PPC-treated and control groups (Fig. 4A), while that of IL-10-producing CD4+ cells was significantly increased in the PPC-treated group compared to that in the control group (Fig. 4B). These results suggest that IL-10-producing CD4+ cells induced by oral feeding of PPC contribute to the observed reduction of CHS responses. The main function of IL-10 appears to be the prevention of extensive tissue damage after inflammation and infection (Saraiva and O'Garra, 2010). IL-10 is an anti-inflammatory cytokine and has a crucial role in terminating the CHS response (Honda et al., 2011). Duan et al. (2011) reported that orally administrated glucosylceramide increased IL-10 in the spleen at 24 h after the DNFB challenge, while the level of IL-10 in the ears was not different at 24 h after the DNFB challenge. We also measured the IL-10 level in the ears of PPC-treated and control groups by ELISA at 24 h after the second challenge in the 0.15% DNFB challenge experiment, and no significant differences were observed between the two groups (data not shown).
Ring et al. (2009) demonstrated that the administration of Treg significantly suppressed the ear swelling response and inflammatory cell infiltration into the skin. These suppressive effects were mediated by IL-10. In addition, Treg from IL-10-deficient mice failed to suppress the CHS reactions by inhibiting leukocyte infiltration into the inflamed skin (Ring et al., 2011) Furthermore, oral administration of the heat-killed Lactobacillus acidophilus strain L-92 upregulated the number of Treg cells in the spleen and cervical lymph nodes, thereby suppressing the progression of DNFB-induced CHS in mice (Shah et al., 2012). These results indicate the importance of Treg and IL-10 to reduce CHS responses. In the present study, we focused on the contribution of IL-10-producing CD4+ cells to reduce CHS responses, and hence the role of Treg in the PPC-treated group was unknown. Future studies should explore this potential role of Treg in CHS responses.
In summary, our data provide evidence that oral feeding of PPC suppresses CHS and antigen-specific IgG1 production in BALB/c mice. The number of splenic IL-10-producing CD4+ T cells was increased by oral feeding of PPC. These results suggest that pomegranate juice consumption may be of therapeutic use for ACD.
Acknowledgements The authors thank the staff of the Medical BioResource Research Unit, and Molecular Cell Biology Research Unit in the Central Research Institute of Kawasaki Medical School. This work was supported by the Japanese Ministry of Agriculture, Forestry and Fisheries and JSPS KAKENHI under Grant number 25350112.