2018 Volume 24 Issue 1 Pages 55-62
2018 Volume 24 Issue 1 Pages 55-62
The drying curves of pullulan, alginate, and blend films at 40, 50 and 60°C were investigated using gravimetric methods. Several mathematical models available in the literature were fitted to the experimental data. The Midilli- Kucuk model satisfactorily described drying kinetics of tested films with the highest R2 and the lowest RMSE, SSE values. For the pullulan-alginate samples, the drying rate increased monotonically with the increasing drying temperature and alginate content of resulting films. However, the heat and mass transfer coefficients of all samples were affected by the temperature during the constant-rate period. Among the temperature tested, effective diffusion coefficient values of tested samples increased progressively with increasing alginate content during the first falling-rate period, whereas this tendency was not observed during the second. Information obtained from this study is essential for designing equipment and optimizing drying parameters for pullulan-alginate edible films.
Edible films are stand-alone sheets of material which can provide a barrier to mass transfer (moisture and oxygen) within the food itself or between the food and environment (Bourlieu et al., 2009; Bourtoom, 2008). Generally, they are prepared from polysaccharides, proteins, lipids or combinations of these components. Among them, the polysaccharide-based films seem to be most attractive, due to their distinctive mechanical and gas barrier properties at low relative humidity (Prommakool et al., 2011). Pullulan, a water-soluble microbial polysaccharide, consists of maltotriose units interconnected to each other via α-(1,6) glycosidic bonds (Sing et al., 2008). Sodium alginate, a natural polysaccharide, composed of variable proportions of β-D-mannuronic acid (M block) and α-L-guluronic acid (G block) linked by 1–4 glycosidic bonds (Zhong et al., 2010). Both pullulan and sodium alginate dissolved in water to form homogeneous film-forming solutions, which upon drying can yield the coherent films that have a wide range of food and pharmaceutical applications (Comaposada et al., 2015; Farris et al., 2014).
Drying process plays an important role in the manufacture of biopolymer films, and their quality often depends on the drying conditions and efficiency. Moreover, improper drying conditions (high temperature or a long time) may create a variety of drying-induced defects such as blisters, warping, and cracks (Islam, 2008). On the basis of Cai and Chen (2008), kinetic analysis of this process is vital for optimization of drying parameters and performance improvements of the drying systems. Therefore, several empirical and semi-empirical models, available in the literature for explaining drying kinetics of polymer or edible films, have been used. The drying kinetics of pregelatinized starch and chestnut starch/carrageenan films were modelled successfully by Karapantsios (2006) and Moreira et al. (2011). Moreover, the thin-layer drying model was developed to explain convective drying kinetic of chitosan-based edible films (Srinivasa et al., 2004). Wong et al. (2004) developed a mathematical model to predict the drying kinetic of poly(vinyl alcohol) films. Additionally, a model for one-dimensional water diffusion was used to fit the experimental drying data of poly(ethylene oxide) and poly(propylene oxide) films (Gu and Alexandridis, 2005). Although the drying kinetics of many polymer films have been reported, such information for pullulan-alginate blended films is currently not available.
Moreover, drying is a complex thermal process, involving simultaneous heat and moisture transfer phenomena (Maroulis et al., 1995). During this process, the heat (hc) and mass (Kc) transfer coefficients are regarded as the key parameters to describing the energy level of moisture molecules, whereas effective moisture diffusivity coefficient (Deff) is related to mechanism of moisture diffusion (Srikiatden and Roberts, 2006). Moreira et al. (2011) studied the variation of hc and Deff values for glycerol-plasticized chestnut starch/carrageenan (CSC) films during drying. The values of hc and Deff for glycerol-plasticized CSC films were lower than that for unplasticized films, due to the formation of strongly hydrogen bonds between glycerol and CSC. During the constant-rate period, Kc values of low-concentration alginate films (1%–3%) decreased with increasing drying temperature, whereas the opposite trend for Deff values were observed during the following falling-rate period (Wong et al., 2014).
The objectives of this study are: (1) to investigate the drying kinetics of pullulan, alginate and blend films dried at 40, 50 and 60°C; (2) to elucidate hc, Kc, Deff and activation energy (Ea) of tested samples as the drying process progressed. In this study, the Midilli-Kucuk model satisfactorily described drying kinetics of the four films. Moreover, their hc, Kc and Ea values were only affected by the temperature during the drying process.
Materials Pullulan (TCI-P0978, MW=2.698×105 g/mol) was purchased from TCI Chemicals Lab. Inc. (Tokyo, Japan). Sodium alginate A2158 (MW=1.806×105 g/mol) was obtained from Sigma- Aldrich Co. Shanghai (Shanghai, China). Redistilled water was used in the preparation of all solutions.
Preparation of samples Pullulan and sodium alginate powders (4%, w/w) were mixed at four pullulan:alginate weight ratios (100:0, 60:40, 40:60, and 0:100), dispersed in distilled water and mixed by a magnetic stirrer (DF-101B, Yueqing, Zhejiang, China) to form a homogeneous film-forming solution.
Drying experiments The film-forming solutions were poured into glass Petri dishes (internal diameter = 7.6 cm), and dried in the environment chamber (PQX-300A, Ningbo Southeast Instrument Company, Ningbo, China) maintained at three temperatures (40, 50 and 60°C, respectively) and the air flow rate of 0 m/s. Moisture loss was periodically recorded during drying by an electronic balance (ME54, Mettler Toledo, Shanghai, China) with a precision of 0.00001 g. The drying process was continued until no further changes of weight were observed. Three replications of each sample were performed, and the data given was an average of these results.
Data analysisMoisture ratio The moisture ratio (MR) of pullulan-alginate-based solutions during drying is calculated as Eq. (1):
 |
Where m0 is the initial moisture content (g H2 O/g DM), me refers to the equilibrium moisture content (g H2O/g DM), and mi is the moisture content at time t (g H2O/g DM).
Mathematical modelling of drying curves The resulting MR data were fitted to the selected mathematical models (Table 1). Non-linear regression was chosen to obtain the parameters in each of the selected models using SAS software (Statistical Analysis System Inst. Inc., Cary, NC, USA) based on the Levenberg-Marquardt method. The coefficient of determination (R2) is one of the primary criteria to evaluate the fit quality of these models. In addition to R2, the root mean square error (RMSE) and the sum of square errors (SSE) are used to determine the suitability of the fit. The higher values of R2 and the lower values of RMSE and SSE were chosen for goodness of fit (Midilli and Kucuk, 2003). The R2, RMSE and SSE can be calculated as follows:
 |
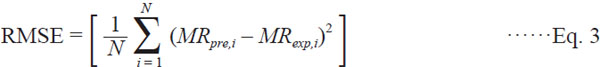 |
 |
| No. | Model name | Model | References |
|---|---|---|---|
| 1 | Page | Two | Page (1949) |
| 2 | Henderson and Pabis | MR=a exp(−kt) | Henderson and Pabis (1961) |
| 3 | Two term | MR=a exp(−k0t)+b exp(−k1t) | Henderson (1974) |
| 4 | Midilli-Kucuk | MR=a exp(−ktn)+bt | Midilli and Kucuk(2003) |
Where MRexp,i is the experimental moisture ratio, MRpre,i is the predicted moisture ratio, and N is the number of data points.
Drying rate The drying rates (DR) were calculated based on the following Eqs. (5) – (7) as used by Guiné et al. (2006) in their studied.
Drying rate at t = t0, (First-order forward finite difference)
 |
Drying rate at t = ti; i=1,....., N-1, (Second-order centered finite difference)
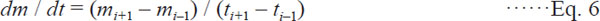 |
Drying rate at t = tN, (First-order backward finite difference)
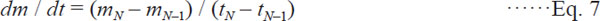 |
where t is drying time (sec), m0 and mN are the moisture content (g H2O/g DM) at initial and equilibrium conditions, respectively.
Heat and mass transfer coefficient during constant-rate period Since the surface of the sample was continuously saturated with free water during the CR period, the drying rate was regarded as evaporation rate of liquid (Maria Elena and Guillermo Hector, 2008). Thus, hc and Kc could be determined using Eqs. (8) and (9):
 |
 |
where kc is the mass transfer coefficient (g.m−2.s−1.Pa−1), As is the area of interface for heat and mass transfer (m2), ms is the mass of the dry solid (g), and Pws and Pw represent the water vapor partial pressure at the surface of the material and at the air (Pa), respectively; hc is heat transfer coefficient (W.m−2.K−1), Tg is the gas temperature (°C), Twb is the surface material temperature (°C), and ΔHv is the heat of vaporization of water at the drying temperature (J/mol).
Effective diffusivity coefficient during the falling-rate period To quantitative analysis of moisture diffusion during the FR period, Fick's second law shown in Eq. (10) was used to describe the Deff (Maria Elena and Guillermo Hector, 2008).
 |
where Deff represents an effective transport coefficient (m2/s), z is the spatial coordinate.
Assuming one-dimensional moisture movement without volume change, constant diffusivity, uniform initial moisture distribution, and negligible resistance, the Eq. (10) can be transformed to the following equation.
 |
where L is thickness (m).
For a long drying period, the above equation simplifies only to first term (Ade-Omowaye et al., 2003). The simplified Eq. (12) takes logarithmic form as followed:
 |
A straight line is obtained from Eq. (12) by plotting ln MR versus drying time, and the Deff for each sample can be calculated from the slope as Eq. (13).
 |
Generally, Deff value can be related with temperature by following the Arrhenius-type relationship:
 |
where D0 is the pre-exponential factor of Arrhenius equation (m2/s), Ea is the activation energy (kJ/mol), R is the universal gas constant (8.314 J/mol); and T is the absolute temperature (K).
Statistical analysis All experiments were conducted in triplicate. Analysis of variance (ANOVA) procedures was used to analyze data, using SAS software (Statistical Analysis System Inst. Inc., Cary, NC, USA).
Drying kinetics and modeling The variation of MR with drying time for pullulan, alginate and blend films dried at 40, 50 and 60°C are presented in Fig. 1. As shown, the MR values for all samples decreased exponentially as the films solidified. Similar results have been reported for chitosan, alginate and chestnut starch/carrageenan films (Moreira et al., 2011; Srinivasa et al., 2004; Wong et al., 2014). Moreover, the drying time of the four tested samples decreased gradually with increasing temperature from 40 to 60°C, due to an increase in heat transfer (Kaya et al., 2008). Among the temperatures tested, pure pullulan samples exhibited the highest MR value and pure alginate had the lowest, suggesting that the drying rate of pullulan samples was lower than that of alginate.

Drying curves of pullulan, alginate and blend films dried at (a) 40°C, (b) 50°C, (c) 60°C. (Solid lines represent the Midilli-Kucuk model fit to the data).
To determine the MR as a function of drying, four mathematical models (Table 1) were applied to fit the experimental data. Meanwhile, the values of R2, RMSE, and SSE for pullulan-alginate samples determined using nonlinear regression analysis are summarized in Table 2. Within the temperature range examined, the Midilli-Kucuk model represented the drying kinetics of pullulan-alginate samples with the highest R2 and the lowest RMSE, SSE values. Also, the predicted drying curves, based on the Midilli-Kucuk model, are presented as continuous lines in Fig.1. These observations indicated that the Midilli-Kucuk model made a better prediction than the Page, Henderson and Pabis and Two term model, and satisfactorily described the drying kinetics of pullulan, alginate and blend films.
| Models | Samples | Temperature (°C) | R2 | RMSE | SSE |
|---|---|---|---|---|---|
| Page | Pullulan | 40 | 0.995 | 0.026 | 0.016 |
| Pul:Alg(60:40) | 0.994 | 0.028 | 0.018 | ||
| Pul:Alg(40:60) | 0.995 | 0.023 | 0.012 | ||
| Alginate | 0.998 | 0.015 | 0.005 | ||
| Pullulan | 50 | 0.993 | 0.031 | 0.022 | |
| Pul:Alg(60:40) | 0.993 | 0.029 | 0.018 | ||
| Pul:Alg(40:60) | 0.995 | 0.026 | 0.015 | ||
| Alginate | 0.995 | 0.024 | 0.012 | ||
| Pullulan | 60 | 0.994 | 0.025 | 0.018 | |
| Pul:Alg(60:40) | 0.997 | 0.028 | 0.020 | ||
| Pul:Alg(40:60) | 0.996 | 0.022 | 0.011 | ||
| Alginate | 0.994 | 0.026 | 0.013 | ||
| Henderson and Pabis | Pullulan | 40 | 0.958 | 0.075 | 0.136 |
| Pul:Alg(60:40) | 0.959 | 0.073 | 0.124 | ||
| Pul:Alg(40:60) | 0.965 | 0.067 | 0.093 | ||
| Alginate | 0.971 | 0.062 | 0.084 | ||
| Pullulan | 50 | 0.949 | 0.083 | 0.167 | |
| Pul:Alg(60:40) | 0.956 | 0.074 | 0.109 | ||
| Pul:Alg(40:60) | 0.952 | 0.080 | 0.148 | ||
| Alginate | 0.959 | 0.074 | 0.109 | ||
| Pullulan | 60 | 0.961 | 0.063 | 0.115 | |
| Pul:Alg(60:40) | 0.949 | 0.083 | 0.173 | ||
| Pul:Alg(40:60) | 0.966 | 0.067 | 0.112 | ||
| Alginate | 0.952 | 0.077 | 0.114 | ||
| Two term | Pullulan | 40 | 0.993 | 0.029 | 0.020 |
| Pul:Alg(60:40) | 0.992 | 0.029 | 0.019 | ||
| Pul:Alg(40:60) | 0.991 | 0.030 | 0.019 | ||
| Alginate | 0.995 | 0.024 | 0.013 | ||
| Pullulan | 50 | 0.992 | 0.029 | 0.021 | |
| Pul:Alg(60:40) | 0.997 | 0.018 | 0.006 | ||
| Pul:Alg(40:60) | 0.988 | 0.040 | 0.037 | ||
| Alginate | 0.991 | 0.034 | 0.026 | ||
| Pullulan | 60 | 0.994 | 0.023 | 0.015 | |
| Pul:Alg(60:40) | 0.987 | 0.038 | 0.035 | ||
| Pul:Alg(40:60) | 0.993 | 0.031 | 0.023 | ||
| Alginate | 0.988 | 0.040 | 0.030 | ||
| Midilli-Kucuk | Pullulan | 40 | 0.998 | 0.013 | 0.004 |
| Pul:Alg(60:40) | 0.998 | 0.133 | 0.004 | ||
| Pul:Alg(40:60) | 0.999 | 0.009 | 0.002 | ||
| Alginate | 0.999 | 0.010 | 0.002 | ||
| Pullulan | 50 | 0.998 | 0.016 | 0.006 | |
| Pul:Alg(60:40) | 0.999 | 0.010 | 0.002 | ||
| Pul:Alg(40:60) | 0.997 | 0.017 | 0.007 | ||
| Alginate | 0.997 | 0.017 | 0.006 | ||
| Pullulan | 60 | 0.998 | 0.014 | 0.005 | |
| Pul:Alg(60:40) | 0.997 | 0.017 | 0.007 | ||
| Pul:Alg(40:60) | 0.999 | 0.009 | 0.002 | ||
| Alginate | 0.998 | 0.012 | 0.004 |
Drying rate Fig. 2 displays the DR curves of pullulan, alginate and blend films dried at 40, 50 and 60°C. As shown, DR values of four samples increased monotonically with increasing temperature and alginate content of the resulting films, suggesting that both drying temperature and composite of sample affected the evaporation rate of moisture. Three distinct drying periods, including warming-up (WU), constant-rate (CR) and falling-rate (FR) period, were noticeably exhibited in Fig. 2a–c. Similar curve shapes have been reported for pure alginate films, chestnut starch/carrageenan, and starch-fiber films (Moreira et al., 2011; Oliveira de Moraes et al., 2015; Wong et al., 2014). At the beginning of drying, the initial increase in DR values could be caused by transference of sensible heat from surrounding environment to the samples (Al Hodali, 1997). After the short WU period, the surface of samples was continuously saturated with free water by capillary flow. As a result, the drying behavior of the samples was essentially similar to the evaporation of pure liquid. Theoretically, DR value would remain constant throughout the CR period (Maria Elena and Guillermo Hector, 2008). However, the actual DR data in this study were observed to fluctuate slightly (Fig. 2). This could be attributed to the phase transition of moisture between the sample surface and surrounding environment (Wang et al., 2007). To the end of CR period, the MR value reached the critical moisture content value (Mcr). After that, the surface of a sample was no longer saturated with moisture, thereby decreasing the DR values as the drying process progressed. As presented in Fig. 2, two well-defined FR periods we observed for pullulan, alginate and blend samples. Among the temperatures tested, DR values of all samples in the first falling-rate (FR1) period were higher than that in the second (FR2). Similar phenomena were observed in the drying process of sodium caseinate/starch films (Ettelaie et al., 2013). This result might imply that the different species of bonding water were removed during the FR1 and FR2 periods. From the previous two-dimensional FTIR spectroscopy study, three identified species of water, which were water with strong hydrogen bond (AW-S), water with moderately hydrogen bond (AW-M) and free water (FW), respectively, existed during the drying process of pullulan-alginate films. The AW-S species exhibited the lowest evaporation rate among all three water species investigated (Xiao et al., 2012). In summary, we can conclude that FW species were firstly evaporated in the CR period, followed by AW-M species in the FR1. Finally, the AW-S species tend to be removed in the FR2 period.

Drying rate of pullulan, alginate and blend films dried at (a) 40°C, (b) 50°C, (c) 60°C.
Mcr values of pullulan, alginate and blend films dried at 40, 50 and 60°C were also illustrated in Fig. 2. Incorporation alginate into pullulan films significantly increased the Mcr value of the composite samples in the given temperature range tested. It could be explained that the alginate was more hydrophilic, which is consistent with the moisture sorption results from our previous study (Xiao et al., 2012). As shown in Fig. 2a–c, Mcr values of pure pullulan samples dried at 40, 50 and 60°C were 6.352, 6.655 and 7.098 g H2O/g DM, respectively, while that gradually increased with increasing drying temperature. According to the ANOVA analysis, considerable variation in Mcr values of pullulan films was observed (p<0.05). In contrast, in the range of experimental temperature tested, these differences of Mcr values for pure alginate and blend samples were not statistically significantly (p>0.05). These observations suggested that Mcr value of pullulan samples was more sensitive to temperature change, whereas this tendency was not observed for pure alginate and blend samples.
Evaporation mass and heat transfer coefficient during constant-rate period To quantify the moisture and energy transfer during the CR period, the evaporation Kc and hc values of pullulan-alginate samples were summarized in Fig. 3 and 4. For four tested samples, the Kc values did not show a clear difference with increasing drying temperature from 40 to 50°C. However, as drying temperature increased from 50 to 60°C, a considerable decrease in Kc values was observed. In contrast, the hc values of all samples tended to increase with increasing drying temperature (Fig. 4). This is consistent with the previous drying curves results shown in Fig. 1; it has been reported that temperature had a positive effect on the rate of heat transfer. Within the temperature range tested, a weak dependence of Kc and hc values on the sample composition was exhibited in Fig. 3 and 4, indicating that the moisture and heat transfer of pullulan-alginate samples were remarkably affected by drying temperature during the CR period.

Kc for pullulan, alginate and blend films dried at 40, 50 and 60°C.

hc for pullulan, alginate and blend films dried at 40, 50 and 60°C.
Effective diffusivity coefficient during falling-rate period Effective diffusivity is used to characterize the transport mechanism of internal moisture during the FR period, which includes molecular diffusion, liquid diffusion, vapor diffusion, unsaturated capillary flow, Knudsen diffusion, and hydrodynamic flow (Karathanos et al., 1990). Usually, Deff is regarded as the parameter to describe the transport rate of internal moisture during this drying period (Al Hodali, 1997). As shown in Fig. 5 (a) and (b), the values of Deff1 and Deff2 for four samples increased obviously with increasing drying temperature, suggesting that the transfer rate of internal moisture was accelerated as the temperature increased. However, Deff1 values for all samples were much higher than the values of Deff2 within the temperature range from 40 to 60°C, indicating that a larger amount of energy should be utilized to transfer the AW-S from samples. This is consistent with the previous results of DR curves (Fig. 2); it has been reported that the DR values for four samples in the FR1 period were higher than that in the FR2 period. As increasing alginate content from 0 to 100%, the Deff1 values of tested samples increased progressively among the temperatures tested, whereas the Deff2 values did not show a clear trend with the changing in alginate content. These results could be attributed to the difference in transport mechanism of internal moisture during the FR1 and FR2 periods.

(a) Deff1, and (b) Deff2 for pullulan, alginate and blend films dried at 40, 50 and 60°C.
The values of Ea for pullulan, alginate and blend samples during the RF1 and RF2 drying periods were estimated in Table 3. As presented, the Ea value of pullulan, alginate and blend samples in the FR1 period were 25.678, 33.137, 26.151 and 26.883 KJ/mol, respectively, which were significantly lower than that of all samples in FR2 periods (45.979, 54.128, 42.516 and 36.305 KJ/mol, respectively). These results not only indicated that Ea values of four tested samples in the FR period were independent of their compositions, but also suggested that the higher-energy amount for all samples was required to evaporate the AW-S during the FR2 period.
| Ea, KJ/mol | ||
|---|---|---|
| Samples | FR1 period | FR2 period |
| Pullualn | 25.68 ± 0.41 | 45.98 ± 1.22 |
| Pul:Alg(60:40) | 33.14 ± 0.58 | 54.13 ± 1.76 |
| Pul:Alg(40:60) | 26.15 ± 0.46 | 42.52 ± 2.22 |
| Alginate | 26.88 ± 0.51 | 36.31 ± 2.19 |
Drying characteristics of pullulan, alginate, and blended films at 40, 50 and 60°C were experimentally determined and modelled. The Midilli-Kucuk model gave the best prediction of the drying kinetics of four films within the temperature range examined. According to the shape of DR curves, two well-defined FR periods were observed for pullulan-alginate samples. Moreover, the DR values of all samples in the FR1 period were higher than that in the FR2, indicating that different species of water were removed during the FR1 and FR2 periods. During the CR period, Kc values of tested films decreased gradually with increasing drying temperature from 50 to 60°C, whereas the opposite trends for hc values were observed. This indicated that the rate of heat and mass transfer for pullulan-alginate films was significantly affected by drying temperature. During the FR period, the Deff values of four tested films increased progressively as the temperature increased. Similar trends of Deff1 values were detected with increasing the alginate content from 0 to 100%. Moreover, Ea value of pullulan, alginate and blend samples in the FR1 period were significantly lower than that of all samples in FR2 periods. The drying characteristics knowledge obtained from this study not only lays a theoretical foundation for designing equipment and optimizing drying parameters for pullulan-sodium alginate based edible films, but also helps to predict the performance of the drying systems.
Acknowledgments The authors gratefully acknowledge the financial support from the Natural Science Foundation of China (No. 31401658, No. 31401679), the Natural Science Foundation of Hunan Province (2017JJ3115), and “1515 Talents Program” of the Hunan Agricultural University.
moisture ratio
R2coefficient of determination
RMSEroot mean square error
SSEsum of square errors
CSCchestnut starch/carrageenan
DRdrying rate
CRconstant-rate drying periods
FR1first falling-rate drying periods
FR2second falling-rate drying periods
Deffeffective diffusion coefficient
Deff1effective diffusion coefficient in the first falling-rate period
Deff2Effective diffusion coefficient in the second falling-rate period, Mcr, critical moisture content
DRcdrying rate at constant-rate period
kcmass transfer coefficient
hcheat transfer coefficient
AW-Swater with strong hydrogen bond
AW-Mwater with moderately hydrogen bond
FWfree water with monomeric non-hydrogen bond
Eaactivation energy