2018 Volume 24 Issue 1 Pages 97-103
2018 Volume 24 Issue 1 Pages 97-103
A simple, rapid and efficient method for the determination of picoxystrobin in watermelon ecosystem was established and validated via QuEChERS (quick, easy cheap, effective, rugged and safe) method using rapid resolution liquid chromatography tandem mass spectrometry (RRLC-MS/MS). The average recoveries (n=5) of picoxystrobin in three matrix at three fortification levels ranged from 89.4% to 102.6%. The limits of detection (LODs) were lower than 1.28×10−4 mg L−1. The limits of quantification (LOQs) were 0.001 mg kg−1 for watermelon flesh (whole watermelon) and 0.005 mg kg−1 for soil. The half-life of picoxystrobin of whole watermelon (soil) in Shandong and Anhui were 1.43 (4.18) days and 3.71 (17.32) days, respectively. The picoxystrobin terminal residues in watermelon were lower than 0.001 mg kg−1 under the recommended dosages, which were far below the maximum residue limit (MRL) set by China (0.05 mg kg−1). These obtained data not only provide scientific information about safety estimation of picoxystrobin in watermelon and the kinetics of dissipation, but also facilitate trade exportation of watermelon for China.
Strobilurin pesticides with high-stability and effectiveness have been widely applied to agricultural field for the prophylaxis and regulation of fungal crop pathogens since its introduction (Howell et al. 2014). These pesticides are synthetic analogues of the β-methoxyacrylate compounds produced by the fungus Strobilurus tenacellus and Oudemansiella mucida (Esteve-Turrillas et al. 2010). Picoxystrobin, as a new broad-spectrum fungicide (Wang et al. 2014), was first announced by Syngenta in 2000 (Esteve-Turrillas et al. 2010), and then was ratified in the European Union in 2003 and enrolled at the U.S. Environmental Protection Agency in 2012. Picoxystrobin acts its fungicidal activity by inhibiting the mitochondrial respiration of fungi (Dornellas et al. 2009; Mercader et al. 2012; Esteve-Turrillas et al. 2010; Sauter et al. 1999). The chemical structures of picoxystrobin are shown in Fig. 1.
Chemical structure of picoxystrobin
Watermelon is the third most popular fruit and vegetable worldwide (Guner and Wehner 2004), especially in summer. However, the yield was affected by the threat from anthracnose, powdery mildew, fusarium wilt et al. (Everts and Himmelstein 2015; Keinath and DuBose 2004). Frequent applications of picoxystrobin are necessary and common since watermelon needs a long growing period (more than 80 days) (Nabavi-Pelesaraei et al. 2014), however, the inappropriate use may cause the fungicide residue in watermelon ecological environment. So, it is essential to assess its terminal residues and dissipation kinetics in the open-fields.
Up to now, the residue of picoxystrobin has been concentrated in analytical methods in different matrices, including crops (Walorczyk et al. 2015; Mercader et al. 2012; Guan et al. 2013; Hiemstra and Kok 2007; Cunha and Fernandes 2011), baby foods (Viñas et al. 2009), fruits and vegetables (Lacina et al. 2010; Guan et al. 2014; Melo et al. 2012; Campillo et al. 2010), fruit juice (Liang et al. 2013; You et al. 2015), honeybees (Walorczyk and Gnusowski 2009), beer (Esteve-Turrillas et al. 2010), and chrysanthemum (Xue et al. 2015). In these studies, gas chromatography-mass spectrometry (GC-MS) was the most commonly used instrument (Bartlett et al. 2002; Walorczyk et al. 2015; Cunha and Fernandes 2011; Melo et al. 2012; You et al. 2015). Gas chromatography-electron capture detector (GC-ECD) (Liang et al. 2013; Esteve-Turrillas et al. 2010), high-performance liquid chromatography (Campillo et al. 2010; Liang et al. 2013), ultra performance liquid chromatography/time of flight mass spectrometry (UPLC-TOF-MS) (Lacina et al. 2010) have also been introduced in the analysis of picoxystrobin. As far as we know, no paper was published on the analysis methods, dissipation behaviour of picoxystrobin in watermelon and soil by rapid resolution liquid chromatography triple quadrupole tandem mass spectrometry (RRLC-QqQ-MS/MS). The safety evaluation of picoxystrobin was firstly reported in this study.
This study was designed to build an uncomplicated and high-efficient QuEChERS (quick, easy, cheap, effective, rugged and safe) methodology for the extraction and quantitative determination of picoxystrobin in watermelon ecosystems using rapid resolution liquid chromatography tandem mass spectrometry (RRLC-MS/MS). At the same time, we analyzed and detected the actual samples of three representative sites (Shandong and Anhui) by using the established method. The dissipation dynamics of picoxystrobin in watermelon flesh, soil and the whole watermelon were studied to obtain the half-life data. Our work will be conducive to provide scientific information about the dissipative dynamics and picoxystrobin terminal residues.
Reagents Picoxystrobin (99.0%) was purchased from Dr. Ehrenstorfer GmbH (Germany). Two hundred fifty g L−1 picoxystrobin suspension concentrate (SC) was supplied by Qingdao Hansen Biologic Science Co, Ltd (Qingdao, China). Purified water was prepared by a Milli-Q water purification system. NaCl, MgSO4, analytical-grade acetonitrile and acetic acid were purchased from Beijing Chemical Reagents Company (Beijing, China). Primary secondary amine (PSA, 40–60 µm), graphitized carbon black (GCB, 40–60 µm) were provided by Tianjin Bonna-Agela Technologies (Tianjin, China). HPLC-grade acetonitrile was purchased from Thermo Fisher Co, Ltd. (MA, USA).
Field Trails The field experiments were carried out in Laiyang (Latitude 36.97°N, Longitude 120.64°E, east of China, semiarid continental monsoon climate), Suzhou (Latitude 34.18°N, Longitude 116.93°E, mid-east of China, temperate continental monsoon climate zone), Shandong province and Anhui province during August to October in 2014, respectively. The soil characteristics of Suzhou and Laiyang were as follows: Suzhou, sandy loam, organic matter 1.71%, pH 6.8; Laiyang, clay loam, organic matter 3.89%, cec 16.7 cmol kg−1, pH 7.32. The experiments were devised on the basis of NY/T 788–2004 (Guideline on Pesticide Residue Trials). Five experimental treatments including one control treatment with 30 m2 were presented and each processing performed three repetitions. Buffer zones were set to isolate different treatment zones. During the experimental period, the average temperature was 26°C (Suzhou) and 27°C (Laiyang), respectively. The average precipitation was 186 mm (Suzhou) and 167 mm (Laiyang).
To research the decay of picoxystrobin in whole watermelon and soil, the commercial picoxystrobin (250 g L−1 SC) was sprayed once on the surface of the black soil and watermelon with three replicates at the dosage of 281.25 g a.i. ha−1 (1.5 times of the recommended high dosage). The control plots were only sprayed with water. Representative soil and whole watermelon samples were randomly collected from three replicate plots at 0 (2h after spraying), 1, 3, 5, 7, 14, 21 and 30 days after application. About 1 kg soil (0–10 cm depth) and 2 kg watermelon samples were collected randomly at different spots. Watermelon and soil samples were preserved at −20°C in deep cryogenic refrigerator.
A low dose level of 187.5 mg a.i. kg−1 and a high dose level of 281.25 mg a.i. kg−1 were used in final residue test plots dealt with 3 and 4 times in order to study the terminal residues of picoxystrobin, respectively. The representative soil (1 kg), watermelon flesh (2 kg) and whole watermelon (2 kg) sample were randomly collected at Pre-Harvest Interval (PHI) of 3, 7, 14 and 21 days. Then they were preserved in a hypothermic chamber at −20°C.
RRLC-MS/MS analysis The RRLC-MS/MS system included an Agilent 6420 Triple Quad coupled to an Agilent 1260 Infinity Binary liquid chromatography pump, a 1260 automatic sampler, a vacuum degasser, and a ZORBAX C18 column ( Agilent Technologies Inc., Santa Clara, CA, USA), which was used for chromatographic separation. The instrument parameters are listed in Table 1.
| RRLC | MS/MS | Ion quantification | Ion confirmation | ||||
|---|---|---|---|---|---|---|---|
| column | Agilent ZORBAX SB-C18 3.0 mm × 50 mm, 2.7 µm | Desolvation gas temperature (□) | 350 | Ionization mode | ESI positive | Ionization mode | ESI positive |
| Mobile phase | water with 0 .1% formic acid (A) acetonitrile (B) A:B = 80:20 | Desolvation gas flow (L min-1) | 10 | Capillary voltage (V) | 4000 | Capillary voltage (V) | 4000 |
| Column temperature (□) | 30 | Nebulizer gas pressure (psi) | 35 | Fragmentor (V) | 80 | Fragmentor (V) | 80 |
| Flow rate (mL min-1) | 0.45 | Collision energy (V) | 19 | Collision energy (V) | 3 | ||
| Injection volumn (µL) | 5 | Precursor ion (m/z) | 368 | Precursor ion (m/z) | 368 | ||
| Run time (min) | 2 | Product ion (m/z) | 145 | Product ion (m/z) | 205 | ||
Sample pretreatment The whole watermelon, blank soil and watermelon flesh samples were collected from the open field for the fortification experiments and matrix effect study. Samples were chopped with a kitchen knife. The prepared samples were preserved in the dark at −20°C. Soil (2.0 g), whole watermelon (2.0 g) and watermelon flesh (2.0 g) were weighed into 50 mL polytetrafluoroethylene centrifuge tubes, spiked with picoxystrobin standard solution (for the matrix matched study, picoxystrobin was added after the clean-up procedure). Whole watermelon and watermelon flesh were extracted with 10 mL acetonitrile (v/v) and vortexed for 1 min, while soil samples were mixed with 2 mL water and 10 mL acetonitrile and vortexed for 1 min. After adding 4 g MgSO4 and 1 g NaCl to the centrifuge tubes, shake the sample vigorously for 1 minute, and let it be centrifuged for 3 minutes at 4000 rpm. After the extraction procedure, 1 mL soil supernatant was moved to a 2 mL polytetrafluoroethylene (PTFE) tube containing 150 mg MgSO4 and 100 mg PSA. For whole watermelon and watermelon flesh samples, 1 mL extract-solutions were purified with 100 mg PSA, 5 mg GCB and 150 mg MgSO4, and then shake for 1 min until the supernatant was almost colorless. After that, the tubes were centrifuged for 3 min at 1000 rpm to stratify completely. Prior to analysis, the upper cleaning fluid was filtered by a 0.22 µm filter into the automatic injector.
Recovery assay Recovery tests were carried out at three fortification levels in five parallel treatments (whole watermelon and watermelon flesh: 0.001, 0.01, 0.1 mg kg−1; soil: 0.005, 0.01, 0.1 mg kg−1). The samples were then processed as described above. Blank samples were performed following a similar procedure to inspect matrix interference.
Optimization of pre-treament#x00A0; Picoxystrobin is a non-polar, weak basic compound. When formic acid was added into acetonitrile as extractant, recovery percentage for soil was about 40% and 30% for whole watermelon and watermelon, which is lower than those in the case of acetonitrile without formic acid as extractane. Hence, acetonitrile was top priority. Previous studies have reported that for the QuEChERS method the addition of water in some dried matrices such as soil samples was recommended to increase the extraction efficiency (Xue et al. 2014; Kolberg et al. 2011; Zhao et al. 2012; González-Curbelo et al. 2011; Xu et al. 2014). In this study, recoveries of the analytes were at the acceptable range (80–110%) for soil samples after adding 2 mL water in acetonitrile.
The dispersive solid-phase extraction (d-SPE) method, with the superior features of simplified, fast and low-costs, was adopted in this study. PSA, GCB, C18, florisil and neutral alumina are commonly used as sorbents (Wilkowska and Biziuk 2011; Balinova et al. 2007; Lehotay et al. 2005). PSA has a weak anion exchange function and is usually used for the removal of various polar organic acids, polar pigments, some sugars and fatty acids from the non-polar sample (Wilkowska and Biziuk 2011; You et al. 2014). GCB with weaker polarity is commonly used for the removal of sterols and pigments such as chlorophyll and carotenoids (Balinova et al. 2007; You et al. 2014). The choice of dispersed solid phase adsorbents depends on the polarity of the pesticide and type of the matrix (Xue et al. 2014). We achieved a content purification effect through purifying the soil sample by pressure swing adsorption. Nevertheless, there was no obvious effect for the whole watermelon and watermelon samples with PSA. Several researches have shown that GCB pigments adsorption was more powerful than PSA (You et al. 2014; Ge et al. 2011). At this stage, different amounts of GCB (i.e., 3, 5, 8, 10 mg) were used to purify the whole watermelon and watermelon flesh samples. As shown in Fig. 2, the recoveries were below 80% when 8 mg or 10 mg GCB was mixed with 100 mg PSA were used as the d-SPE sorbents. Previous studies have shown that exceed 5 mg GCB was used for the separation and purification of complicated samples when the recovery rate was less than 70% (Ge et al. 2014). The sorbents of 3 mg GCB or 5 mg GCB with 100 mg PSA showed good recoveries, but 5 mg GCB performed better pigment cleaning properties than 3 mg GCB. Therefore, 5 mg GCB mixed with 100 mg PSA was chosen to clear up the whole watermelon and watermelon flesh samples.
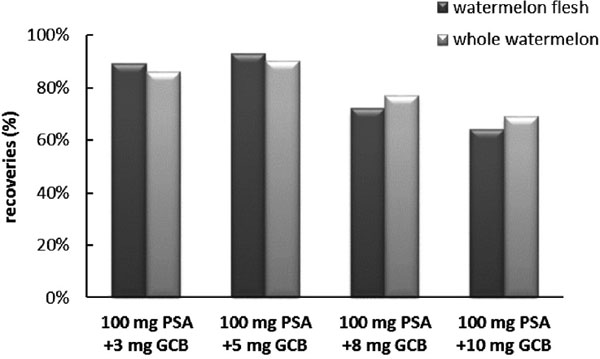
Recoveries of picoxystrobin in watermelon flesh and whole watermelon with different amount of sorbe
Method validation The quantitative errors of the matrix effects were checked by the matrix-matched calibration (Lagunas-Allué et al. 2012). Different concentration levels of picoxystrobin (ranging from 0.001–0.5 mg kg−1) were added to the blank extractants of soil, whole watermelon and watermelon flesh. The calibration curves were obtained by plotting the peak area of the target analyte against the concentration in those three matrices. Good linearity was achieved for picoxystrobin at the six concentration levels with the correlation coefficients (r) higher than 0.999. The regression equations for picoxystrobin in soil, watermelon flesh and whole watermelon are given in Table 2.
| Samples | Calibration curve | r | LODs (mg L−1) |
|---|---|---|---|
| Watermelon flesh | y = 36500x + 278.62 | 0.9991 | 1.03×10−4 |
| Whole watermelon | y = 45785x + 187.68 | 0.9990 | 5.77×10−5 |
| Soil | y = 69673x + 78.747 | 0.9999 | 1.28×10−4 |
The enhanced recoveries were studied in five replicate samples of three concentrations to verify method validity. The recovery values of picoxystrobin in soil samples were 89.4% to 96.4%, with the relative standard deviation (RSDs) ranging from 7.3% to 12.1% (Table. 3). Simultaneously, the recoveries ranged from 92.4% to 102.6% and 91.6% to 99.0%, respectively, with the RSDs of 2.9–12.2% and 3.9–13.2% for whole watermelon and watermelon flesh samples. The limits of quantification (LOQs) were defined as acceptable minimum concentrations for accuracy and accuracy determination (MacDougall and Crummett 1980). The LOQs of picoxystrobin were 0.001 mg kg−1. The limits of detection (LODs) were usually calculated at a signal-to-noise (S/N) ratio of 3 (Cheng et al. 2014), and the LODs of the method for soil, watermelon flesh, and whole watermelon were 1.28 × 10−4, 1.03 × 10−4 and 5.77 × 10−5 mg L−1, respectively.
| Samples | Fortified level (mg/kg) | Average recovery (%) | RSD (%) | LOQ (mg/kg) |
|---|---|---|---|---|
| 0.005 | 96.4 | 10.8 | ||
| Soil | 0.05 | 95.6 | 12.1 | 0.005 |
| 0.5 | 89.4 | 7.3 | ||
| 0.001 | 92.4 | 2.9 | ||
| Whole watermelon | 0.01 | 102.6 | 11.3 | 0.001 |
| 0.5 | 95.4 | 12.2 | ||
| 0.001 | 91.6 | 3.9 | ||
| Watermelon flesh | 0.01 | 93.4 | 13.2 | 0.001 |
| 0.5 | 99.0 | 7.0 |
Dissipation of picoxystrobin under the open fields Under the open field conditions, the residues of picoxystrobin in whole watermelon and soil at different intervals were detected after the application. The initial concentrations of picoxystrobin of whole watermelon in Shandong and Anhui were 0.236 and 0.035 mg kg−1 respectively. After 10 d, more than 99% and 95% of the initial residual were eliminated in Shandong and Anhui, respectively. Thereafter no residue was found in whole watermelon. The initial concentrations of picoxystrobin in soil were 1.922 and 0.164 mg kg−1 in Shandong and Anhui, respectively. At the end of the sampling period, the residue was at 0.008 mg kg−1 and 0.043 mg kg−1 in Shandong and Anhui respectively. The half-life and dissipation kinetics equations for picoxystrobin are summarized in Table 4. Compared with Anhui, picoxystrobin degraded more quickly for both matrixes in Shandong. It might be affected by the climate, soil characteristics, moisture content and physicochemical property of the biocide.
| Matrix | Locality | Regression equation | Determination coefficient (R2) | Half-lifes (d) |
|---|---|---|---|---|
| Soil | Shandong | C = 0.8003e−0.166t | 0.9262 | 4.18 |
| Anhui | C = 0.1385e−0.040t | 0.9419 | 17.32 | |
| Whole melon | Shandong | C = 0.1002e−0.485t | 0.8887 | 1.43 |
| Anhui | C = 0.0208e−0.187t | 0.9087 | 3.71 |
Terminal Residue of Picoxystrobin in Watermelon and Soil Terminal residues of picoxystrobin after the last recommended dosage and 1.5 times of recommended dosage application in Shandong and Anhui in 2014 were shown in Table 5. The results showed that at PHI (pre-harvest intervals) of 3, 7, 14 and 21 days, picoxystrobin residue in soil were 0.012–0.106 mg kg−1 in Shandong and Anhui province, and there were no detectable picoxystrobin residue in watermelon flesh and whole watermelon. Picoxystrobin dissipated slower in soil than in watermelon under field conditions. The maximum residue limit (MRL) of picoxystrobin in watermelon has been set for 0.05 mg kg−1. Due to the terminal residues of picoxystrobin in all matrixes were well below the MRL, we recommend that picoxystrobin (250 g L−1 picoxystrbin SC) can be applied in watermelon at a dosage of 187.5–281.25 g a.i. ha−1, a PHI of at least 3 days was recommended.
| Sample location | Dosage (g a.i.ha−1) | Number of times sprayed | Interval (days) | Residue (mg kg−1) | ||
|---|---|---|---|---|---|---|
| Watermelon flesh | Whole watermelon | Soil | ||||
| Shandong | 187.5 | 3 | 3 | <LOQ | <LOQ | 0.013 ± 0.005 |
| 7 | <LOQ | <LOQ | 0.012 ± 0.007 | |||
| 14 | <LOQ | <LOQ | 0.027 ± 0.014 | |||
| 21 | <LOQ | <LOQ | 0.014 ± 0.118 | |||
| 4 | 3 | <LOQ | <LOQ | 0.056 ± 0.018 | ||
| 7 | <LOQ | <LOQ | 0.016 ± 0.007 | |||
| 14 | <LOQ | <LOQ | 0.028 ± 0.001 | |||
| 21 | <LOQ | <LOQ | <LOQ | |||
| 281.25 | 3 | 3 | <LOQ | <LOQ | 0.027 ± 0.005 | |
| 7 | <LOQ | <LOQ | 0.036 ± 0.006 | |||
| 14 | <LOQ | <LOQ | 0.034 ± 0.005 | |||
| 21 | <LOQ | <LOQ | 0.013 ± 0.021 | |||
| 4 | 3 | <LOQ | <LOQ | 0.106 ± 0.007 | ||
| 7 | <LOQ | <LOQ | 0.055 ± 0.004 | |||
| 14 | <LOQ | <LOQ | 0.044 ± 0.013 | |||
| 21 | <LOQ | <LOQ | 0.042 ± 0.005 | |||
| Anhui | 187.5 | 3 | 3 | <LOQ | <LOQ | 0.041 ± 0.037 |
| 7 | <LOQ | <LOQ | 0.039 ± 0.020 | |||
| 14 | <LOQ | <LOQ | 0.020 ± 0.065 | |||
| 21 | <LOQ | <LOQ | <LOQ | |||
| 4 | 3 | <LOQ | <LOQ | 0.034 ± 0.071 | ||
| 7 | <LOQ | <LOQ | 0.047 ± 0.022 | |||
| 14 | <LOQ | <LOQ | 0.035 ± 0.033 | |||
| 21 | <LOQ | <LOQ | <LOQ | |||
| 281.25 | 3 | 3 | <LOQ | <LOQ | 0.036 ± 0.028 | |
| 7 | <LOQ | <LOQ | 0.041 ± 0.013 | |||
| 14 | <LOQ | <LOQ | 0.036 ± 0.035 | |||
| 21 | <LOQ | <LOQ | <LOQ | |||
| 4 | 3 | <LOQ | <LOQ | 0.066 ± 0.017 | ||
| 7 | <LOQ | <LOQ | 0.057 ± 0.045 | |||
| 14 | <LOQ | <LOQ | 0.042 ± 0.047 | |||
| 21 | <LOQ | <LOQ | <LOQ | |||
In this study, a simple, rapid and efficient method for the determination of picoxystrobin in whole watermelon, watermelon flesh sample and soil by DSPE-RRLC-MS/MS has been developed. The method satisfied the requirement of linearity, repeatability, accuracy and precision with lower LOQ (0.001 mg kg−1) and LOD (5.77 × 10−5 mg L−1). It provides excellent performance and makes it suitable for fast picoxystrobin supervising. The results showed that fungicide half-life ranged from 4.18 to 17.3 days in soil and 1.43 to 3.71 days in watermelon. In watermelon fresh, the residual terminal of picoxystrobin was much lower than that of MRL, which indicated that residue of picoxystrobin in watermelon was not a problem when applied at the recommended dosage.
Acknowledgements This work was supported by financial support from National Natural Science Foundation of China (Project NO. 21677009).
The authors declare no competing financial interest and human conflicts.