2018 Volume 24 Issue 3 Pages 413-420
2018 Volume 24 Issue 3 Pages 413-420
Greeneye (Chlorophthalmus albatrossis) in soaking liquids such as tap water, salty water (SW), vinegar water, salty vinegar water, and salty vinegar broth (SVB) was processed by medium high hydrostatic pressure at medium high temperature (MHHP+MHT; 100 MPa, 65°C, 30 min) or high hydrostatic pressure at low temperature (HHP+LT; 600 MPa, 10°C, 5 min). Both treatments sufficiently inactivated endogenous fish microflora, however MHHP+MHT treatment partly degraded the fish. The suitability of HHP+LT treatment to extend shelf life under refrigeration was further studied since this process preserved the fish shape. The fish with or without head and viscera was immersed in SW or SVB and treated by HHP+LT. Greeneye without head and viscera in SVB was processed by HHP+LT and stored for 3 months, and its suitability for deep-frying was evaluated as high in terms of microbial safety, shape, and palatability.
The family name Chlorophthalmidae (Aulopiformes) derives from the Greek words chloros (green) and ophthalmos (eye). The common name of this family is “greeneye” in English and mehikari in Japanese, both of which are named after their characteristic eyes reflecting light with shining green (Hirakawa et al., 2007). Greeneye are found in tropical and subtropical seas worldwide at upper continental slope depths (Bineesh et al., 2014). Some varieties such as C. borealis, C. albatrossis, and C. acutifrons are caught along the Pacific coast of Japan (Nakabo, 2000). Greeneye species used to be discarded as by-catch by offshore bottom trawl fisheries. Recently, its demand as a food fish has increased and these species have become commercially important for offshore bottom trawl fisheries along the Pacific coast of Japan (Yoshida, 2003). Although the life cycles of greeneye has not been clarified sufficiently, their commercial value has been increasing and they are commonly distributed to rural and urban cities in Japan (Abe, 2009) since its white flesh with moderate fat and edible soft bones contribute to the palatability. Greeneye in Japan are consumed mostly as deep-fried, dried, and broiled and rarely as raw (sashimi in Japanese). However, refrigeration storage of fresh greeneye is limited up to about 2 days and thus most greeneye are distributed as frozen materials for further cooking at home and restaurants. In addition, a time-consuming and laborious thawing process is required for the frozen materials before cooking, often leading to loss in taste and/or nutrients (Gambuteanu et al., 2013). On the other hand, some ready-to-fry seasoned and frozen greeneye products are commercially available in Japan. However, the product variety is limited. Therefore, cooks have requested greeneye as a refrigerated material for cooking.
The shelf life of refrigerated foods can be extended by high hydrostatic pressure (HHP) processing (Yamamoto, 2017). In heat processing, flavor, color, and nutrients are often lost, and unfavorable off-flavors can be produced due to accelerated chemical reactions. In contrast, HHP may suppress chemical reactions and retain the fresh attributes of foods while inactivating microbes. HHP food processing was commercialized in 1990 and the nonthermal process is often carried out at 600 MPa and low temperatures of 5°C–10°C for processing juice, meat products, ready-to-eat meals, dairy products, and so forth. In addition, medium HHP (MHHP) of 100MPa–200 MPa can be combined with medium high temperatures (MHT) of 55°C–75°C to process fruit compotes (Nakaura, 2017).
The effect of HHP treatment on fish and shell fish has been studied for minimizing the risk of microbial growth during cold storage of fresh fish or marinated fish, while maximizing the fresh attributes of fish (Tabilo-Mnunizaga et al., 2016). HHP levels of 400 MPa–600 MPa may denature the fish flesh and the fresh texture can be lost (Tabilo-Mnunizaga et al., 2016).
In this study, HHP treatment of fish was studied to evaluate its applicability to process fish into material destined for further cooking, in which the final products do not require a fresh fish texture. Greeneye (C. albatrossis) in several soaking liquids was processed either by MHHP in combination with medium high temperature (MHHP+MHT) or HHP in combination with low temperature (HHP+LT) to access the processing suitability for extended refrigeration storage of the fish as a cooking material. In addition, the processed fish samples were deep-fried to evaluate their cooking suitability.
Materials Greeneye landed at Gamagori (Aichi, Japan) was cooled in ice cubes and, thereafter, the head and viscera were removed by bare hands with a knife (hereafter, −HV) or left intact (hereafter, +HV). The fish samples were then refrigerated at 5°C until further use or frozen at −20°C. The commercially available frozen samples (−HV) were thawed in a refrigerator at 5°C overnight and used for further study. All the samples after refrigeration storage were washed; they were soaked in tap water in a bowl, stirred sufficiently, and then drained on a screen. The washing process was repeated 10 times.
Liquid preparation As soaking liquids, 4 kinds of liquids were prepared. Commercial table salt (4.0 g; Cooking salt, The Salt Industry Center of Japan, Tokyo, Japan) was dissolved in tap water (100.0 mL) for salty water. A portion (23.8 mL) of commercial cooking vinegar (Cereal vinegar, Mizkan, Aichi, Japan) was diluted with tap water to 100.0 mL. Salty vinegar water was prepared by adding salt (4.0 g) to the vinegar water (100 mL). A mixture of table sugar (7.1 g; white sugar, Nissin Sugar, Tokyo, Japan), the vinegar (23.8 mL), commercial soy sauce (28.6 mL; Dark soy sauce, Kikkoman, Chiba, Japan), sweet Sake (7.2 mL Hon-mirin, Takara Shuzo, Kyoto, Japan), and broth (33.3 mL), which was a solution of powdered fish broth (2.0 g; Hon-dashi, Ajinomoto, Tokyo, Japan) in 300 mL of hot tap water, was boiled and then cooled to room temperature as the salty vinegar broth (Japanese margination sauce, Namban-zu), also known as Japanese marinating liquid. Tap water was also used as a soaking liquid.
HHP treatments (MHHP+MHT and HHP+LT) An equal weight of soaking liquid was added to the washed greeneye in a retort plastic pouch, which was then vacuum-heat-sealed and refrigerated. HHP treatments of MHHP+MHT (100 MPa, 65 °C, 30 min) and HHP+LT (600 MPa, 10 °C, 5 min) were carried out using an MHHP processor (TFS2-500, Toyo Koatsu, Hiroshima, Japan) and an HHP processor (TFS6-50, Toyo Koatsu, Hiroshima, Japan), respectively. The treated sample pouches were immediately transferred to an ice bath and then refrigerated at 5 °C. Each treatment was carried out in at least duplicate. Each trial was performed in triplicate for samples for storage.
Microbial test The unprocessed fish sample before or after the washing procedure was minced in sterile saline of the same weight. The obtained suspension was plated on standard agar (Standard method agar “Nissui”, Nissui Pharmaceutical, Tokyo, Japan) plates directly and/or after serial dilutions. An aliquot (250 µL) of each soaking liquid was directly plated on the agar plates. The HHP-treated sample (fish in each soaking liquid) was minced and plated on the agar plates directly and/or after serial dilutions. Each diluted sample was plated in at least triplicate in all cases. The plates were incubated at 30 °C for a maximum of 4 d until the colony forming units (CFU) reaches a constant value.
Characterization of soaking liquids Each of the fish immersion liquids in pouches was characterized for pH (pH meter D-52, Horiba, Kyoto, Japan), Brix (Pocket Refractometer PAL-1, ATAGO, Tokyo, Japan), and salt concentration (Digital salt meter ES-421, ATAGO, Tokyo, Japan) before and after HHP treatment.
Deep-fry cooking suitability HHP-treated and stored fish were deep-fried to access its suitability as a refrigerated cooking material. As a typical cooking method, the fish was drained, covered with potato starch (Katakuriko, Hokuren, Hokkaido, Japan), and deep-fried in plant oil (Salad oil, Nisshin Oillio Group, Tokyo, Japan) at 180 °C for a few minutes. The appearance of the deep-fried sample was noted. Furthermore, the palatability of the sample was evaluated only after the microbial safety was confirmed by the total viable count of the minced suspension before deep-frying being <2 log CFU/mL. The palatability evaluation was performed by 5 voluntary panelists without knowledge of the sample identities.
Microbial status of washed fish and soaking liquids Reduction of the microbial population before food processing, including HHP food processing, is fundamental for maximizing the intervention effect of processing and minimizing the risk of quality deterioration during storage. Therefore, commercially available frozen −HV greeneye was thawed as described above and the effect of intensive 10 rounds of washing using tap water on the microbial reduction was evaluated. Before the washing process, a total viable count of 4.95 ± 0.07 log CFU/mL was detected with the refrigeration thawed sample, and the count was reduced to 3.33 ± 0.02 log CFU/mL after washing. The washing process was effective in reducing the microbial count. No microbes were detected in salty water, vinegar water, salty vinegar water, and salty vinegar broth, but in tap water, which gave a count of 2.81 ± 0.14 log CFU/mL. There might be synergetic effects between chlorine in tap water and the salt or vinegar on microbial inactivation in salty water, vinegar water, and salty vinegar water, despite not being heat pasteurized.
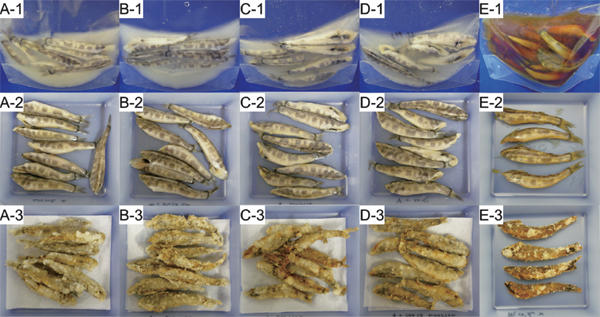
Applicability of HHP treatments to greeneye processing Applicability of HHP treatments (MHHP+MHT and HHP+LT) was investigated by using commercial frozen −HV greeneye. Thawed and washed fish was soaked in each soaking liquid and subjected to each treatment. In all the samples after MHHP+MHT treatment, the liquid became turbid (A-1 to E-1 of Fig. 1) and the fish body partly degraded with its outer skin partly lost, as shown in Fig. 1 A-2 to E-2. In the case of HHP+LT treatment, the fish body shape and its outer skin were retained (A-2 to E-2 of Fig. 2), although the liquid was turbid in all the samples (A-1 to E-1 of Fig. 2). No microbes were detected (< 10 CFU/mL) in all the samples stored overnight at 5 °C after MHHP+MHT or HHP+LT treatment (Table 1). Therefore, all the samples treated by MHHP+MHT or HHP+LT were further subjected to deep-frying. Partly degraded fish bodies after MHHP+MHT treatment retained their partly degraded shape even after deep-frying (A-3 to E-3 of Fig 1). Of all samples, the fish processed in salty vinegar broth by MHHP+MHT (E-3 of Fig. 1) showed better shape retention than the samples in the other liquids and the slightly degraded fish shape was similar to its untreated sample. Since the fish body shape was not altered drastically by deep-frying in all cases, both MHHP+MHT and HHP+LT treatments might be appropriate to retain the fish body shape for deep-fried products.

Greeneye processed in soaking liquid (A – E) by medium high hydrostatic pressure (MHHP) at medium high temperature (MHT).
(A), tap water; (B), salty water; (C), vinegar water; (D), salty vinegar water; (E), salty vinegar broth; (1) processed; (2), drained; (3) deep-fried.
Greeneye processed in soaking liquid (A – E) by high hydrostatic pressure (HHP) at low temperature (LT). (A), tap water; (B), salty water; (C), vinegar water; (D), salty vinegar water; (E), salty vinegar broth; (1) processed; (2), drained; (3) deep-fried.
| soaking liquids | processes | fish shape degradation | total viable counts after 1-day refrigeration | overall palatability | palatability descriptions |
|---|---|---|---|---|---|
| tap water | MHHP+MHT | +++ | N < 10 CFU/mL | poor | watery; bland |
| HHP+LT | − | poor | |||
| salty water (SW) | MHHP+MHT | +++ | good | appetizing due to suppressed fishy smell | |
| HHP+LT | − | good | |||
| vinegar water | MHHP+MHT | +++ | fair | firm flesh; a bit too sour | |
| HHP+LT | − | fair | |||
| salty vinegar | MHHP+MHT | +++ | fair | firm flesh; a bit too sour | |
| HHP+LT | − | fair | |||
| salty vinegar broth (SVB) | MHHP+MHT | + | excellent | fully acceptable; imperceptibly soft bones | |
| HHP+LT | − | excellent |
As for the palatability evaluations, similar comments were obtained from all the panelists. Tap water as the soaking liquid gave an unfavorable watery or bland sensation to the fish. It is speculated that water impregnated the fish body to dilute the endogenous salts, and the salts in the body leached out into the water. Fish in salty water was evaluated as tasty with a reduced fishy smell. The possibility that salt addition might have contributed to masking the fishy smell is supported by the function of salt in fish muscle, which is to increase the osmotic pressure and firm the tissue, thereby preventing certain fishy smelling compounds from leaching out into the surrounding liquid (Ueyanagi, 1987). Panelists rated the vinegar water and salty vinegar water as producing a negative sensation of excess sourness, even after deep-frying. However, the fish body was evaluated as firm and elastic. This phenomenon can be understood based on a speculated synergetic effect of salt and vinegar, which might either harden the muscle fibers via insolubilized aggregated proteins or induce aggregation of hydrophilic proteins in part as glue-like materials among muscle fibers (Shimomura et al., 1984). Some panelists suggested a need to adjust the liquid formulations to make them less sour. Salty vinegar broth was evaluated as the best soaking liquid since the bones became imperceptibly soft with a good salty and sour taste.
Table 1 summarizes the results of microbial safety, fish body shape, and palatability. Microbial safety after one day was confirmed in all the samples, and well-retained fish body shape and acceptable palatability after deep-frying were confirmed for the greeneye samples treated with HHP+LT in salty water (hereafter, SW) or salty vinegar broth (hereafter, SVB).
Therefore, it was decided to study the effect of refrigeration storage on the qualities of those samples and their suitability as deep-fry materials. Refrigerated greeneye samples of +HV and −HV were used in the storage study, assuming a potential application where the caught and landed greeneye would be cut to remove the head and viscera, washed using tap water, soaked in SW or SVB, and stored in a refrigerator after HHP+LT treatment. The HHP+LT processed samples were refrigerated at 5 °C and stored for a maximum of 3 months. Samples were evaluated every month in terms of soaking liquid characterization, microbial test, cooking suitability, and palatability.
Changes in soaking liquid during cold storage after HHP+LT treatment The soaking liquid in each pouch was evaluated before and after HHP treatment and during refrigeration storage in terms of pH, Brix, and salt concentration (Fig. 3).

Changes in (A) pH, (B) Brix, and (C) salt concentration of soaking liquids (SW, salty water; SVB, salty vinegar broth). Greeneye with the head and viscera (+HV) or without (−HV) was treated in each liquid by HHP+LT and refrigerated up to 3 months.
●, +HV in SW; ■, −HV in SW; ○, +HV in SVB; □, −HV in SVB.
SW and SVB before HHP treatment were slightly alkali (∼pH 10) and acidic (∼pH 4), respectively (Fig. 3A). After treatment, the pH values approached approximately pH 7 and pH 5, respectively, in both +HV and −HV samples. The pH values for all liquids did not change significantly during storage, although there was a slight decreasing trend in the case of +HV in SW.
Brix (refractive index), which is often used to estimate sugar concentrations in liquid foods, can also be used as an index for estimating the content of solid components in liquid. Brix values for SVB decreased after 1 d, while those for SW did not change significantly. However, the Brix value of +HV in SW increased after storage for 1 month (Fig. 3B).
Salt concentrations (Fig. 3C) of SW and SVB decreased after 1 d to ca. 2.8%. During storage, a significant change was not observed for all cases. However, it seems that a slight decreasing trend was observed for +HV in SW during storage and for +HV in SVB after 3-month storage, although these may not be significant.
The changes in the characteristic parameters (pH, Brix, and salt concentration) for SW and SVB after 1 d may indicate homogenization of solute concentrations between the fish body and each soaking liquid. The increase in Brix of +HV in SW during storage (1–3 months) may indicate some chemical changes.
Microbiological aspects during cold storage after HHP+LT treatment The effect of the washing process on total viable counts of fresh greeneye after refrigerated distribution was studied using +HV and −HV fish samples before and after washing. Total viable counts for +HV and −HV samples before washing were 5.75 ± 0.04 and 4.77 ± 0.03 log CFU/mL, and these were reduced by washing to 4.28 ± 0.02 and 3.98 ± 0.04 log CFU/mL, respectively (Table 2). In this study, the intensive washing process reduced the counts by ca. 1 log CFU/mL of unwashed fish samples, both +HV and −HV samples, as well as in the refrigeration thawed fish. The counts were higher in +HV sample than in −HV, and this is attributed to the fact that gills and digestive tracts had been removed in −HV samples. The gills and digestive tracts are often contaminated with microbes (Shewan, 1961), and they may not be washed thoroughly by the washing procedure in this study.
| pre-processing | refrigeration period after HHP+LT processing | ||||||
|---|---|---|---|---|---|---|---|
| fish | soaking liquid | before wash | after wash | 1 d | 1 month | 2 months | 3 months |
| +HV | − | 5.84 ± 0.04 | 4.28 ± 0.02 | − | − | − | − |
| SW | − | − | ND | ND | 1.53 ± 2.28 | ND | |
| SVB | − | − | ND | ND | ND | ND | |
| −HV | − | 4.81 ± 0.11 | 3.98 ± 0.04 | − | − | − | − |
| SW | − | − | 0.95 ± 0.31 | 6.16 ± 0.57 | 6.25 ± 0.72 | 6.11 ± 0.35 | |
| SVB | − | − | ND | ND | ND | ND | |
As for the soaking liquids, it was confirmed that trace microbes (N < 4 CFU/mL) and no microbes (N < 1 CFU/mL) were detected from SW and SVB, respectively.
Microbial behavior during storage after HHP+LT processing of greeneye in the SW or SVB was monitored at one-month intervals for up to 3 months (Table 2). From the stored sample of −HV in SW, trace microbes (ca. 1 log CFU/mL) were detected after 1 d storage. A high number of microbes of ca. 6 log CFU/mL was detected after 1 month. In contrast, total viable counts of the sample +HV in SW were much less than that of −HV in SW at each storage time, although the +HV sample retained a possible source of microbial contamination: the head and viscera. Since the head and viscera had been removed by bare hands with a knife in the −HV samples, this process might have caused unintended microbial contamination from the worker's hands or other sources. In addition, it should be noted that with the 2-month storage of +HV fish in SW, microbes were undetected from two pouches and detected from one pouch among triplicate trials, resulting in a greater standard deviation. It was suggested that the fish microflora might differ among the pouches. In the +HV and −HV samples soaked in SVB, microbes were not detected (< 10 CFU/mL) in all the samples during storage up to 3 months. It was concluded that the greeneye (+HV and −HV) soaked in SVB was optimal for HHP+LT treatment and subsequent refrigerated storage up to 3 months. Based on a safety factor of 0.7 for shelf life determination, a refrigeration storage period of 2 months may be guaranteed for practical use at least.
Freshly caught fish is often contaminated with Pseudomonas, Alcaligenes, Vibrio, Serratia, and Micrococcus, although the microflora composition depends on the microbial contents of the water in which the fish live (Gram and Huss, 2000). These psychrophilic bacteria are ubiquitous and can grow at refrigeration temperatures (Gram and Huss, 2000). However, in general, most bacteria do not grow under acidic conditions (Jay, 1978). As shown in Table 2, the number of microbes detected in the −HV samples in SW (> 6 log CFU/mL) was much higher than in the +HV and −HV samples in SVB (< 1 log CFU/mL). Psychrophilic microbes in slightly alkaline SW (pH ∼ 10) may have either survived HHP+LT treatment for further growth or become injured and then recovered for growth during cold storage (Koseki et al., 2008). On the other hand, the slightly acidic SVB (pH ∼ 5) may have suppressed the growth of any surviving microbes following HHP+LT treatment. Furthermore, although trace microbes (ca. 1 log CFU/mL) were detected in the sample refrigerated for 1 day after HHP+LT treatment of refrigerated (unfrozen) fish in SW (Table 2), microbes were not detected (< 10 CFU/mL) in all the samples after 1-day cold storage following HHP+LT or MHHP+MHT treatment of frozen and thawed fish in any soaking liquids in Table 1. Notably, the microflora may differ between frozen and unfrozen fish (Shewan, 1961), and the varied microbial counts between them might be ascribed to differences in the microflora between frozen and refrigerated greeneye. In any case, HHP+LT treatment inactivated the microbes on/in greeneye by 3–4 log units effectively.
Deep-fry cooking suitability Greeneye was soaked in SW or SVB and sealed in a pouch. The pouch was subjected to HHP+LT treatment and then stored under refrigeration (5 °C) for up to 3 months. In the SW and SVB soaking liquids, the finger feel of the +HV fish samples became softer than that of −HV before deep-frying after 1 month and 2 months, respectively (data not shown). The stored samples were then deep-fried at 180 °C (Fig. 4) to evaluate their cooking suitability and palatability.

Deep-fried greeneye with the head and viscera (+HV) or without (−HV) processed in salty water (SW) or salty vinegar broth (SVB) by high hydrostatic pressure (HHP) at low temperature (LT) and refrigerated for 1 d, 1 month, 2 months, and 3 months.
(A), +HV in SW; (B), −HV in SW; (C), +HV in SVB; (D), −HV in SVB; (0), 1 d; (1), 1 month; (2), 2 months; (3), 3 months.
The shapes of the +HV fish in SW after storage for 1 and 2 months were degraded into small fragments immediately after immersion into the hot oil. The deep-fried fragments were only recoverable from the oil by using a sieve. The deep-fried shape of +HV fish in SW after 3-month storage seemed to retain the fish body shape, but the inner part was lost during the cooking. The inner part of the +HV fish in SW after HHP+LT treatment might have been degraded by autolytic enzymes during storage and intermediate degradation might lead to the fragmented fish body. During the cold storage of +HV fish in SW after HHP+LT treatment, the fish bodies were degraded by deep-frying, although only trace or no microbes were detected (Table 2). There are at least two types of fish spoilage, bacterial and enzymatic spoilage, and the former precedes the latter (Ghaly et al., 2010). Based on the observation that fish degradation was detected in the presence of trace or no microbial activity, this indicates that in this study the fish flesh was degraded by autolytic enzymes during storage rather than microbes. Although HHP treatment may inactivate some enzymes (Oey, 2016), endogenous autolytic enzymes in the greeneye in SW might be insufficiently inactivated by HHP+LT treatment in this study.
As for the cold storage of +HV/-HV fish in SVB, no microbes were detected (< 10 CFU/mL) in all the cases of refrigeration storage. The fish shape after deep-frying was mostly retained, except for the case of +HV fish in SVB after 3-month refrigeration, in which the flesh of deep-fried fish was lost and the outer skin remained (C-3 of Fig. 4). This indicates that slow autolytic degradation was likely in +HV fish in SVB. The liquid pH during the storage was lower (∼ 5) than in SW (pH ∼ 7) (Fig. 3A), since SVB contains vinegar. The acidic SVB may have contributed to the slow autolytic degradation in comparison with the neutral environment in SW.
The deep-fried shape of −HV fish was retained in both SW and SVB. However, only −HV fish in SVB can be applied to HHP+LT treatment and subsequent cold storage to process a cooking material for deep-frying, since no microbes were detected in the fish in SVB during 3-month storage (Table 2).
Palatability of deep-fried greeneye After confirming microbial safety as shown in Table 2, the deep-fried fish samples were subjected to a palatability evaluation. A common foreign body feeling was claimed by all the voluntary panelists (N = 5) for all the deep-fried +HV samples due to hard head and eyes of the fish. The panelists suggested that the palatability would be improved by removing the head and viscera. In addition, it was commonly noticed that the flesh of deep-fried −HV samples was firmer than that of +HV samples. This is likely due to the presence of autolytic enzymes in +HV samples, which softened the fish flesh unfavorably. The taste of fish samples processed in SVB was judged acceptable by all the panelists.
The processing applicability of two treatments, MHHP+MHT and HHP+LT, before refrigerated storage was studied by using commercially available frozen “greeneye” without head and viscera (−HV). The fish was soaked in several liquid preparations and vacuum pouched. The MHHP+MHT treatment was not suitable for deep-fry processing due to obvious flesh degradation, although microbial safety was confirmed after 1-day cold storage. The HHP+LT treatment also ensured microbial safety, and flesh degradation was highly suppressed compared with the MHHP+MHT treatment. In terms of cooking suitability, salted water (SW) and salted vinegar broth (SVB) were selected from the viewpoint of palatability.
HHP+LT treatment of the fish in SW or SVB was further studied for extended shelf life and the suitability for deep-frying. It was concluded that −HV fish in SVB could be refrigerated for 3 months after HHP+LT treatment and that the stored sample was acceptable for deep-frying with good palatability in terms of the flesh firmness and taste. It was also suggested that the head and viscera of the fish be removed to avoid a foreign body feeling. The processed fish for further cooking was safe and palatable during storage for 3 months, and is suggested to provide a 2-month shelf life to the product based on a safety factor of 0.7.
It was suggested to intensively wash the caught fish with tap water, remove the head and viscera, soak in salty acidic liquid SVB, treat with HHP+LT, and refrigerate the processed greeneye to ensure microbiological food safety. From the viewpoint of retaining its fish flesh quality, suppression of autolysis will be indispensable. The head and viscera of the greeneye should be removed to minimize contamination of autolytic endogenous enzymes and the processed fish should be kept under refrigeration before cooking to minimize the enzymatic effects.
The processing method in this study might be applicable to other fish species with various fish microflora in combination with other soaking liquids of sufficiently low pH, which suppresses the growth of spore-forming bacteria and others. Other soaking liquids can be determined by optimizing saltiness and pH with attention to the microbial, quality, cooking suitability, and palatability aspects. Expensive fish varieties and exclusive applications of HHP technology may overcome the processing cost, which is accepted worldwide by meat packers and fresh juice providers as HHP food processors. The processing cost at 600 MPa for a holding time of 3 min is estimated to be between 0.14 (55 L vessel) and 0.071 Euro/kg (525 L vessel) with 5-year amortization, 300 working d/year, 16 h/d, 60% volume efficiency, and costs for wear of parts and utilities (Balasubramaniam et al., 2016).
Acknowledgments The greeneye samples were kindly donated by Mr. Takayuki Kuroda (Manten Co., Ltd.). Ms. Mika Hirose assisted in the deep-frying of the samples. The voluntary contributions of laboratory members in the palatability evaluations are greatly appreciated.