2018 Volume 24 Issue 5 Pages 869-874
2018 Volume 24 Issue 5 Pages 869-874
While many studies have examined the binding of phytate to proteins and/or minerals, little information is available on the number of complex types and combinations of binding molecules. In this study, interactions of phytate with proteins and divalent ions in the soluble fraction of soymilk obtained from the soybean cultivar Tachinagaha were examined using a Sephacryl S-400 HR column. Phosphorus and divalent ions were detected using inductively coupled plasma atomic emission spectroscopy. Phytate was identified as the phosphorus peak digested by phytase. Three phytate peaks were detected in fractions 21, 49, and 53. The phytate in fraction 21, indicating the presence of the largest complex, was co-eluted with proteins and disappeared after proteinase K treatment. The phytate in fraction 49 was co-eluted with iron, while that in fraction 53 was co-eluted with calcium. Our methods have potential applicability for detecting mineral-phytate and protein-phytate interactions.
Phytic acid, also known as inositol hexakisphosphate (IP6), is the principal storage form of phosphorus in many plant tissues. Phytate is a mixed cation salt of phytic acid (Lott et al., 2000) and its metal ion bound form is stored in the globoids of plant seeds (Otegui, 2002). Phytic acid is found as a complex salt of Ca, Mg, and K, and in some cases it is bound to proteins and carbohydrates in mature seeds. These complexes of phytic acid are generally called phytin (Lott, 1984).
Many studies have examined the binding of phytic acid with minerals (inorganic ions) and/or proteins in plants. Energy-dispersive X-ray microanalyses of many plant species have revealed that, other than P, the globoids contain mainly K and Mg as well as low levels of Ca, Mn, Fe, and Zn (Lott, 1984; Lott et al., 1995; Wada and Lott, 1997), indicating that phytate is a mixed salt of these cations. Iwai et al. (2012) suggested that phosphorus translocated from source organs is immediately converted to phytic acid and accumulates in the aleurone layer of rice, with Ca, K and Fe accumulating as phytate in the aleurone layer. In contrast, Zn is bound loosely to phytic acid and accumulates not only in phytate, but also in another storage form. They also suggested that Cu accumulates in the endosperm and may be present in a storage form other than phytate. It has been suggested that phytate occurs primarily as a potassium–magnesium salt in broad beans (Lott and Buttrose, 1978), and as a calcium–magnesium–potassium salt in soybeans (Fontaine et al., 1946). Protein–phytate complexes have been well documented in wheat (Kumar et al., 2010; Hill and Tyler, 1954). The probable existence of a soluble protein–cation–phytate complex in situ in soybean has been demonstrated by gel filtration (Prattley and Stanley, 1982). Phytase treatment altered the protein solubility in defatted soymilk, further indicating that phytate in defatted soymilk may form a protein–phytate complex with β-conglycinin and glycinin (Saito et al., 2001). Ishiguro et al. (2008) examined the amount of phytate bound to particulate and soluble proteins in soymilk.
Phytate is thought to be related to food quality, i.e., nutritional quality, and processing properties. Phytate is stable over a broad pH region as a highly negatively charged ion; therefore, its dietary presence negatively impacts the bioavailability of divalent and trivalent metal ions. In contrast to its negative effects, its antioxidant properties and therapeutic effects have also been reported (Kumar et al., 2010). The effect of phytate on the processing properties of soybean has also been studied. Phosphate groups of phytic acid can buffer the coagulation reaction in soymilk by binding coagulants such as magnesium and calcium (Saio et al., 1969; Toda et al., 2006; Ishiguro et al., 2008). Phytate also binds to soluble and particulate proteins and is incorporated into the particulate proteins, which form a network of tofu curds with the addition of coagulant (Guo and Ono, 2005; Ishiguro et al., 2008). Al Mahfuz et al. (2004) examined the relationship between phytate and astringency in soymilk and concluded that potassium phytate shows astringency, while calcium phytate does not. The form of phytic acid (free or phytate form) and its binding partner may be keys to elucidating the effect of phytic acid or phytate on food quality.
Although many studies have demonstrated the importance of phytate in food quality, little information is available about phytate complexes such as the number of complex types and combinations of binding molecules. In the present study, three types of phytate complexes with divalent ions or proteins were detected by subjecting soymilk to gel filtration. These complexes showed different elution fractions and components. We anticipate that our methods will be applicable to the study of mineral-phytate and protein-phytate interactions and their effects on food quality.
Materials Soybeans (Tachinagaha) were field grown at the National Institute of Crop Science, NARO, Tsukubamirai, Ibaraki, Japan in 2009. After harvesting, the soybeans were stored at 5 °C until use. Experiments were performed by November 2013.
Preparation of soymilk Soybeans (19 g) were washed three times with distilled water and soaked in distilled water at 20 °C for 18 h. The hydrated seeds were drained and ground into a homogenate using a rolling chopper-type homogenizer (AMT-PA2; SMT Co., Ltd., Tokyo, Japan) with distilled water equivalent to six times the weight of dry seed minus absorbed water. Raw soymilk was separated from the homogenate by centrifugation at 3,000 rpm using a centrifugal separator (SYK-3800-15A; Sanyo Rikagaku Kikai Co., Ltd, Tokyo, Japan) equipped with a nylon filter (120 mesh), and was then heated by incubating for 6 min in boiling water. During incubation, the temperature of the soymilk in a test tube (1.5 cm in diameter) increased to 95 °C at 1 min after the start, and was maintained at above 95 °C for 5 min.
Size-exclusion chromatography Soymilk was centrifuged at 20,400 × g for 70 min at room temperature. The supernatant was collected and the cream fraction was removed. Then, 1.1 mL of the supernatant was fractionated on a 1.6 × 60 cm Sephacryl S-400 HR column (HiPrep 16/60 Sephacryl S-400 HR; GE Healthcare Japan Co., Ltd., Tokyo, Japan) at a flow rate of 1 mL/min using Tris buffer (10 mmol L−1 Tris-HCl, 40 mmol L−1 KCl, pH 7.0) as the elution buffer, and 2 mL of each fraction was collected. The bed volume of the column was 120 mL. The void volume was determined using blue dextran 2000. Blue dextran 2000, albumin, and carbonic anhydrase were purchased as a set (GE Healthcare Japan Co., Ltd.) and used as a standard.
Enzymatic treatment Soymilk was treated with 0.1 mg/mL of proteinase K (Nacalai Tesque, Kyoto, Japan) at 37 °C for 4 h, followed by inactivation in boiling water for 5 min. The supernatant from the soymilk was treated with 44 mg/mL of wheat phytase (0.03 U/mg solid; Sigma-Aldrich Japan, Tokyo, Japan) at 40 °C for 2.5 h. We confirmed that incubation at 40 °C for 2.5 h without enzyme did not affect the elution pattern.
Measurements of phytate and minerals Phytate in the soymilk and supernatant was precipitated by adding 20 mmol L−1 CaCl2 (pH 11.5) according to the method described by Ishiguro et al. (2005). The precipitated phytate was redissolved in 0.9 mol L−1 HCl and analyzed by a colorimetric assay using Wade reagent (Gao et al., 2007). Phosphorus, calcium, and iron present in the soymilk, supernatant, and fractions separated by size exclusion chromatography were detected by inductively coupled plasma atomic emission spectroscopy (ICP-AES; Optima 4300DV; PerkinElmer Japan Co., Ltd., Kanagawa, Japan).
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) SDS-PAGE was performed essentially as described by Laemmli (1970) using a 12.5% acrylamide gel (ePAGEL; Atto, Tokyo, Japan).
After centrifugation at 20,400 × g for 70 min, 80% of the phytate in soymilk obtained from the soybean cultivar Tachinagaha remained in the supernatant. The supernatant of soymilk (untreated, treated with proteinase, and treated with phytase) was fractionated on a Sephacryl S-400 HR column at a flow rate of 1 mL/min, and 2 mL of each fraction was collected. Figure 1 shows the elution patterns of the phosphorus obtained from the untreated supernatant, that treated with phytase, and that treated with proteinase K. Three peaks corresponding to phosphorus were detected for the untreated supernatant at fractions 21, 49, and 53, at the elution volumes of 42, 98, and 106 mL, respectively (Fig. 1, arrows). After phytase treatment, the first and the second phosphorus peaks obtained for the untreated sample (detected in fractions 21 and 49, respectively) disappeared, and only one peak was detected in fraction 54; these results indicate that the first and second phosphorus peaks of the untreated supernatant represent the elution of phytate. The peak obtained after phytase treatment was close to the third peak obtained for the untreated supernatant in fraction 53. Nakano et al. (2000) investigated the pathway of dephosphorylation of phytic acid to form myo-inositol using phytase obtained from wheat bran. Based on the structures identified, they showed that the initial hydrolysis of the phosphate ester occurs at the D/L-4 position of phytic acid, followed by the hydrolysis of phosphate esters at the other positions. The optimum pH of wheat phytase is around 5.5, while the pH of the soymilk analyzed in the present study is around 6.5. Brejnholt et al. (2010) showed that the enzymatic activity of wheat phytase sharply decreases as the pH increases above 5.5. The peak obtained in fraction 54 of the phytase-treated sample might represent the incomplete degradation of inositol phosphate. The elution pattern of calcium implies that the third peak observed for the untreated sample in fraction 53 represents the elution of phytate, as explained below. Thus, we can conclude that the three phosphorus peaks represent the elution of phytate; only phytic acid-phosphorus was detected in this study. Our previous result indicates that the ratio of phytic acid-phosphorus to total phosphorus in soymilk was around 0.8 (Toda et al., 2006). It is likely that non-phytic acid-phosphorus was not detected due to the lower concentration.

Fractionation pattern of phosphorus in the supernatant of soymilk for untreated samples (◆), treated with phytase (○), and treated with proteinase K (△) on a Sephacryl S-400 HR column. Arrows indicate the peak of phosphorus of untreated samples. The fraction of the void volume (38 mL) is indicated as V0.
After proteinase K treatment, only the first peak associated with phytate in fraction 21 of the untreated sample disappeared (Fig. 1), indicating protein binding of phytate.
Figure 2A shows the elution patterns of proteins in the untreated supernatant. Protein subunits of glycinin and β-conglycinin were eluted as the main proteins for fraction 21, which coincided with the first phytate peak (Fig. 1). Proteins were almost completely degraded by proteinase K treatment. Only bands less than 25 kDa were detected in fractions 41 to 48 by SDS-PAGE (Fig. 2B).

Fractionation pattern of protein in the supernatant of soymilk for untreated sample (A) and that treated with proteinase K (B) on a Sephacryl S-400 HR column analyzed by SDS-PAGE. M: Protein marker. The sizes (kDa) of the marker are on the left side of the photograph. The fraction numbers are on the upper side of the photograph. Glycinin (A1a, A1b, A2, A3, A4, and basic subunits) and β-conglycinin (α′, α, and β subunits) proteins are indicated.
Albumin (67 kDa) and carbonic anhydrase (29 kDa) as standard proteins were fractionated under the same conditions described above, and their peaks were eluted at fractions 26 and 33, respectively. Thus, fraction 21 probably consists of several protein complexes, some of which are bound to phytate. In addition, interaction among the protein subunits of glycinin and β-conglycinin in soymilk has been reported (Ono et al., 1991; Guo et al., 1997). Further studies are needed to reveal the mechanism by which a protein binds to phytate or the protein molecules constituting phytate–protein complexes.
Figure 3 shows the elution patterns of calcium in the untreated, phytase-treated, and proteinase K-treated supernatants. Two calcium peaks were observed for the untreated supernatant at fractions 21 and 53 (Fig. 3, arrows). The peak for fraction 53 of the untreated sample disappeared after phytase treatment, while the peak for fraction 21 remained. Since the removal of phosphate groups by phytase results in the release of binding cations such as calcium, iron, and zinc (Lei and Porres, 2003), the peak for fraction 53 likely represents the phytate bound to calcium (Figs. 1 and 3). Another possibility is that the peak for fraction 53 represents inositol pentaphosphate (IP5) bound to calcium. It has been reported that in soybean phytic acid contributes 93% of the total inositol phosphate and IP5 contributes only 3% (Vincent et al., 2015), while in soy flakes, 14% of the inositol phosphate is in the form of IP5 (Lehrfeld, 1989). Further studies are needed to identify the peak for fraction 53. After proteinase K treatment, only the calcium peak detected for the untreated supernatant for fraction 21 disappeared. This peak also disappeared when 2.5 mM of EGTA was added in the elution buffer, while the phosphorus peak for this fraction remained (data not shown). These results indicate that phytate and calcium are independently bound to proteins in this fraction.
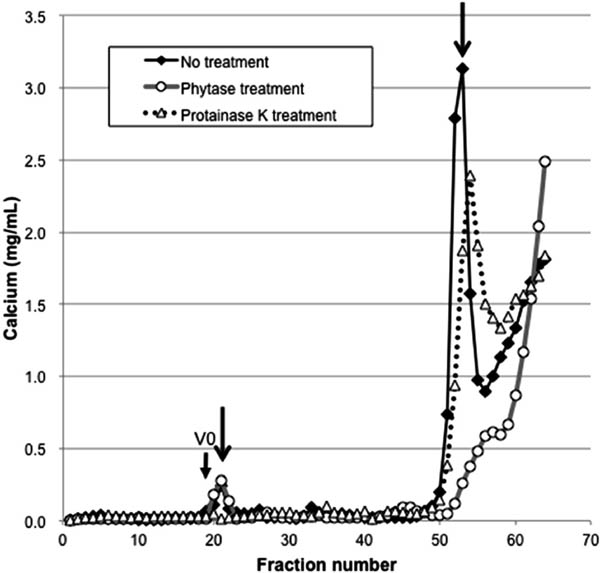
Fractionation pattern of calcium in the supernatant of soymilk for untreated samples (◆), treated with phytase (○), and treated with proteinase K (△) on a Sephacryl S-400 HR column. Arrows indicate the peak of calcium of untreated samples. The fraction of the void volume (38 mL) is indicated as V0.
In the present study, only the protein in particulate form was observed to interact with phytate, while Ishiguro et al. (2008) reported that 38% of phytate in soymilk obtained from the cultivar Suzuyutaka was bound to soluble protein. Buffer conditions such as pH and ionic strength potentially affect the elution pattern. In the present study, with a bed volume of 120 mL, about 60% phosphorus and iron were detected, while less than 20% calcium was detected (data not shown). Since calcium has been reported to form a ternary complex with phytate and protein (Prattley and Stanley, 1982), its behavior needs to be examined under different elution conditions in future studies. Cultivar differences should also be considered.
Figure 4 shows the elution patterns of iron obtained using the untreated, phytase-treated, and proteinase K-treated supernatants. Three iron peaks were detected using the untreated supernatant of fractions 21, 37, and 49 (Fig. 4, arrows). After phytase treatment, only the peak for fraction 49 disappeared and a peak appeared for fraction 53, which is interpreted as a likely shift of the peak for fraction 49 to fraction 53. These results suggest that the phytate in fraction 49 (Fig. 1) is bound to iron and is degraded incompletely by phytase treatment. The iron–phytate peak of the untreated supernatant associated with fraction 49 eluted faster than the calcium–phytate peak of the untreated sample associated with fraction 53 (Figs. 1 and 3). Molecules other than proteins might interact with the iron–phytate complex. Another possibility is that the number of phosphate groups differs. Iron–phosphorus interactions likely remained after phytase treatment in fraction 53 (Figs. 1 and 4), which differed from the calcium–phosphorus interactions that disappeared after phytase treatment (Figs. 1 and 3). Further studies are needed to clarify these discrepancies.

Fractionation pattern of iron in the supernatant of soymilk for untreated samples (◆), treated with phytase (○), and treated with proteinase K (△) on a Sephacryl S-400 HR column. Arrows indicate the peak of iron of untreated samples. The fraction of the void volume (38 mL) is indicated as V0.
After proteinase K treatment, the iron peaks associated with fractions 21 and 37 disappeared, indicating that the iron in these fractions is protein bound; however, because the iron peak in fraction 21 remained after phytase treatment, phytate is likely bound to protein without iron acting as an intermediary.
Magnesium and zinc, which potentially interact with phytate, were also analyzed by ICP-AES; however, their elution patterns were not changed after phytase treatment. One possible reason is the incomplete degradation by wheat phytase. Interactions between phytate and these divalent ions will be analyzed in a future study.
In conclusion, three different phytate complexes were detected in soymilk using size exclusion chromatography and ICP-AES. Phytate was bound to calcium, iron, or protein independently, forming various complexes, which may exert differential effects on processing properties such as coagulation reactivity and taste, or the nutritional quality of soymilk. Analysis of each complex can provide useful information regarding the relationship between phytate and food quality, which has not been fully resolved by simply analyzing the phytate contents of various food items.
Acknowledgement This study was financially supported by the Brand-Nippon project of the Ministry of Agriculture, Forestry and Fisheries.