2018 年 24 巻 5 号 p. 903-910
2018 年 24 巻 5 号 p. 903-910
The aim of the study was to investigate the combinational effect of Pine needle polysaccharide (PNP) and Kudzu flavonoids (KF) on cell differentiation and fat metabolism in 3T3-L1 cells. 3T3-L1 cells were treated with various concentrations of PNP or/and KF. Cell viability, Oil red O staining, intracellular TG, glycerol in culture medium, levels of cytokines and expression of transcription factors genes were evaluated. PNP and KF combination significantly suppressed cells differentiation and fat accumulation by inhibiting the mRNA expression of PPARγ2 (peroxisome proliferator activated receptor-γ2), C/EBPα (CCAAT/enhancer binding proteins-α) and SREBP-1c (sterol regulatory element binding protein-1c) in 3T3-L1 cells. In addition, the gene expression and secretion of leptin and IL-6 were inhibited, adiponectin was significantly increased, secretion of TNF-α was inhibited by PNP and KF combination. Taken together, PNP and KF combination may exert anti-adipogenic effects through regulation of lipid metabolism gene expression of the transcription factors and cytokines. The findings suggested that PNP and KF combination may be explored as a potentially promising food additive for prevention of obesity.
Obesity has been becoming prevalent in both developed and developing countries. Importantly, obesity was associated with an increased incidence of metabolic disorders, especially 2 type diabetes mellitus, cardiovascular disease, hypertension, hyperlipidemia, and cancer (Kopelman, 2007). Overweight and obesity were attributed to combination of over-consumption of energy-rich foods and reduction of physical activity, which led to increase of adipocyte number (hyperplasia) and size (hypertrophy) (Gesta et al., 2007). Therefore, inhibition the differentiation of preadipocytes into adipocytes can reduce the weight of adipose tissue, resulting in a decrease of the occurrence of obesity.
Cedrus deodara is commonly called as deodar, also known as Himalayan cedar, and belongs to the family of Pinaceae (Mahajan et al., 2016). Pine needle parts of C. deodara, it is recognized as a Chinese herbal medicines or healthy food material with abundant protein, vitamins, and minerals for the purposes of health promotion (Zeng et al., 2012), and the extract displays anti-obesity activity (Jeon and Kim, 2006). Pine needle polysaccharides (PNP) were one of major biologically active components of pine needle (Zeng et al., 2014), and polysaccharide as a biological macromolecular had potential effects on decrease of lipid accumulation in adipocytes (Choi et al., 2016). Kudzu root (Gegen in Chinese) was the dried root of Pueraria lobata (Willd.) Ohwi, it was often used as an herbal medicine for the treatment of fever, diarrhoea, diabetes and cardiovascular diseases (Wong et al., 2011). Kudzu flavonoids (KF) was extracted from kudzu root, and significantly reduced body weight gain and the liver lipid level in ovariectomized rats (Lim et al., 2013). In addition, puerarin of principal component in KF has been shown inhibition of adipogenesis and intracellular lipid accumulation in vitro (Wang et al., 2013). A number of studies related to the anti-obesity effect and other biological properties of individual PNP or KF have been carried out. However, the combinational effect of PNP and KF on the adipocytes differentiation and fat accumulation has not been investigated. Therefore, the vitro model was established using 3T3-L1 cells to explore the combinational effect of PNP and KF on lipid metabolism. And obesity-related physiological parameters, the levels of adipocytokines and mRNA expression of transcription factor were investigated.
Materials and reagents PNP (over 90% purity) and KF (over 90% purity, puerarin content 88%) were bought from Nanjing Jingzhu Biological Technology Inc (Nanjing, China). 3T3-L1 preadipocyte cells were purchased from American Type Culture Collection (Virginia, USA). Dulbecco's modified Eagle's medium (DMEM) and dimethyl sulfoxide (DMSO) were purchased from Beijing Solarbio Science & Technology Inc. (Beijing, China). Fetal bovine serum (FBS) was purchased from Biological Industries (Jerusalem, Israel). Insulin, dexamethasone, 3-(4, 5-dimethylthiazol-2-yl) 2, 5-diphenyltetrazolim bromide (MTT), 3-isobutylmethoxylxanthine (IBMX), Oil red O and noradrenaline (NA) were purchased from Sigma (State of Missouri, USA). Leptin, adiponectin, TNF-α, IL-6 enzyme-linked immunoassay (ELISA) kit were purchased from Cloud-Clone Corp (Houston, USA). Tissue triglyceride assay kit and Tissue glycerol assay kit were purchased from Applygen Technologies Inc (Beijing, China).
Cell viability The 3T3L1 preadipocytes were seeded at a density of 4×103 cells/mL into 96-well plates and incubated for 24h until confluency. Then, cells were treated with different concentrations of PNP or/and KF (were dissolved in the medium) for 4 days. Media were changed every 2 days. Next, cells were incubated with MTT (5 mg/mL) solution at 37 °C for 4 h, and the blue formazan formed by living cells was dissolved in DMSO with shaking for 10 min. Then, absorbance was detected at 540 nm by using a microplate reader (Spectramax M2, USA).
Differentiation of 3T3-L1 preadipocytes The 3T3-L1 preadipocytes were grown in DMEM with high glucose containing 10% FBS, 100 U/mL penicillin and 100 µg/mL streptomycin at 37 °C in a humidified atmosphere of 5% CO2. Two days later, 3T3-L1 preadipocytes reached full confluency, followed by addition of differentiation DMEM medium containing 10% FBS, 10 µg/mL insulin, 0.5 mM IBMX, 0.25 µM dexamethasone to induced differentiate (defined as day 0), cell differentiation for another 2 days. Then, the differentiated 3T3-L1 cells were incubated with DMEM medium supplemented with 10 µg/mL insulin for another 2 days. After that the differentiated 3T3-L1 cells were cultured in DMEM medium supplemented with 10% FBS for another 4 days. Various concentrations of PNP and KF were dissolved in the differentiation medium and 3T3-L1 cells were maintained in the differentiation medium for 4 days.
Oil red Ostaining Cells differentiation on 8th day, cells were washed twice with PBS (pH=7.4) twice and fixed with 10% formaldehyde for 1h. Then, cells were incubated with Oil Red O solution (0.6%, prepared in isopropanol) for 1 h at room temperature. After that, the excess Oil Red O dye was washed with PBS (pH=7.4). The stained lipid droplets were observed by inverted microscope (XD-202, China) at magnification× 40. Cells with stained lipid droplets were dissolved in isopropanol and evaluated via absorbance at 540 nm.
Quantification of intracellular TG Cells differentiation on 8th day, the harvested adipocytes were washed with PBS (pH=7.4), collected in cell lysis buffer RIPA (Beyotime, Shanghai, China). After that, cells were centrifuged at 13000×g for 20 min at 4 °C. The suspension was used to measure the intracellular TG by enzymatic kit (Applygen, Beijing, China) according to the manufacturer's instructions.
Quantification of glycerol in culture medium Cells differentiation on 8th day, add or without add 0.1 µg/mL norepinephrine (NA) treatment for 24h. The culture medium were centrifuged at 13000×g for 5 min, the supernatant was used to measure the glycerol content by enzymatic kit (Applygen, Beijing, China) according to the manufacturer's instructions.
ELISA analysis of cytokines Cells differentiation on 8th day, Leptin, adiponectin, TNF-α and IL-6 were assayed in triplicate measurements of culture medium samples. Cytokines in culture medium quantitative assays were determined by ELISA kit (Cloud-Clone Corp, Houston, USA) according to the manufacturer's instructions. Optical densities were measured with a microplate spectrophotometer capable of reading at 450 nm.
Measurement of mRNA expression by RT-qPCR Cells differentiation on 8th day, total RNA was extracted from 3T3-L1 adipocytes using the Trizol Reagent (Invitrogen) according to the manufacturer's instructions. RNA (1 µg) was reverse-transcribed using the cDNA Reverse Transcription Kit (TransGen Biotech, China), according to the manufacturer's protocols. Realtime quantitative PCR was performed using an Applied Biosystems 7900HT Real-Time PCR System (Applied Biosystems, USA) and Premix Ex Taq (Probe qPCR), according to the protocols provided by the manufacturer. The sequences of the primers corresponding to the cell adipogenic genes analyzed in this study are shown in Table 1, β-actin as internal reference. Results are using the 2−ΔΔCT method.
| Gene | Forward | Reverse | Probe |
|---|---|---|---|
| PPARγ2 | 5′-ATGATGGGAGAAGATAAAATCAAGTTC-3 | 5′-GGATGGCCACCTCTTTGCT-3′ | 5′-AACATATCACCCCCCTGCAGGAGCA-3′ |
| C/EBPα | 5′-GCGAGCACGAGACGTCTATAGA-3 | 5′-GCCAGGAACTCGTCGTTGAA-3′ | 5′-ATCAGCGCCTACATCGACCCGG-3′ |
| SREBP1c | 5′-CAACCAGAAGCTCAAGCA-3′ | 5′-AGGTCCTTCAGTGATTTGC-3′ | 5′-AGAACCTGACCCTACGAAGTGCACACAAA-3′ |
| Adiponectin | 5′-GAACTTGTGCAGGTTGGATG-3′ | 5′-TGCATCTCCTTTCTCTCCCT-3′ | 5′-CTGCCATCACGGCCTGGTGT-3′ |
| Leptin | 5′-CAAGCAGTGCCTATCCAGAA-3′ | 5′-GTGAAGCCCAGGAATGAAGT-3′ | 5′-CACGCAGTCGGTATCCGCCA-3 |
| IL-6 | 5′-AGTCCGGAGAGGAGACTTCA-3′ | 5′-ATTTCCACGATTTCCCAGAG-3′ | 5′-CAGAGGATACCACTCCCAACAGACCTG-3′ |
| β-actin | 5′-AGAAAATCTGGCACCACACC-3′ | 5′-CCATCTCTTGCTCGAAGTCC-3 | 5′-TAAAACGCAGCTCAGTAACAGTCG-3′ |
Statistical analysis All values were expressed as means ± standard error (SE). Continuous variables were tested for normality, and then analyzed using one-way ANOVA. Statistical analysis was performed with SPSS 17.0 (SPSS Inc., Chicago, USA). Differences of P < 0.05 were considered to be statistically significant.
Cell viability assay PNP at 0–320 µg/mL were similar with control from the growth profiles (Fig. 1A), but KF at concentrations more than 80 µg/mL resulted in cytotoxicity (Fig. 1B). Therefore, 80, 160 µg/mL of PNP and 20, 40 µg/mL of KF were selected for further study. The results showed that PNP and KF combination treatment group were similar with control group (Fig. 1C) and did not induced cytotoxicity during the proliferation of 3T3-L1 preadipocytes.

Cell viability in 3T3-L1 cells by MTT assay treated with PNP or/and KF for up to 4 days. (A) PNP. (B) KF. (C) PNP or/and KF.
I: control; II: 40 µg/mL KF; III: 160 µg/mL PNP; IV: 20 µg/mL KF+80 µg/mL PNP; V: 20 µg/mL KF+160 µg/mL PNP; VI: 40 µg/mL KF+80 µg/mL PNP; VII: 40 µg/mL KF+160 µg/mL PNP.
All data are presented as the mean ± SE of six independent experiments. Means within the same column but not sharing the same superscript letter are significantly different (P < 0.05)
Effect of PNP and KF combination on fat accumulation in 3T3-L1 cells Compared with control, PNP and KF combination significantly reduced the amounts of lipid droplets (Fig. 2A). Oil red O was extracted from the cells to be shown in Fig. 2B. Apparently, high dose PNP and KF combination most strongly inhibited lipid droplet accumulation. Meanwhile, intracellular TG level was shown in Fig. 2C, the groups of both KF alone and high dose combination were significantly reduced to 80% and 67.8%, respectively. These results suggested that PNP synergy with KF decreased fat accumulation, and KF might play a potent suppressive role in inhibition of 3T3-L1 cells fat accumulation.

Effect of PNP and KF combination on lipid accumulation in 3T3-L1 cells differentiation on 8th day. (A) Oil Red O staining. (B) Oil Red O content. (C) TG level.
I: control; II: 40 µg/mL KF; III: 160 µg/mL PNP; IV: 20 µg/mL KF+ 80 µg/mL PNP; V: 20 µg/mL KF+160 µg/mL PNP; VI: 40 µg/mL KF+80 µg/mL PNP; VII: 40 µg/mL KF+160 µg/mL PNP.
All data are presented as the mean ± SE of six independent experiments. Means within the same column but not sharing the same superscript letter are significantly different (P < 0.05)
Effect of PNP and KF combination on lipolysis in 3T3-L1 cells The effect of PNP and KF combination on lipolysis was studied by detecting glycerol level in culture medium (Fig. 3). Without added NA, all groups showed no significantly difference on glycerol level. However, when the NA was added, glycerol levels in experimental groups were remarkably increased compared with control. Mightily decomposition of PNP and KF combination was better than PNP alone. These results suggested that KF synergy with PNP promoted hydrolysis of TG, and PNP might play a potent suppressive role in lipolysis of 3T3-L1 cells.
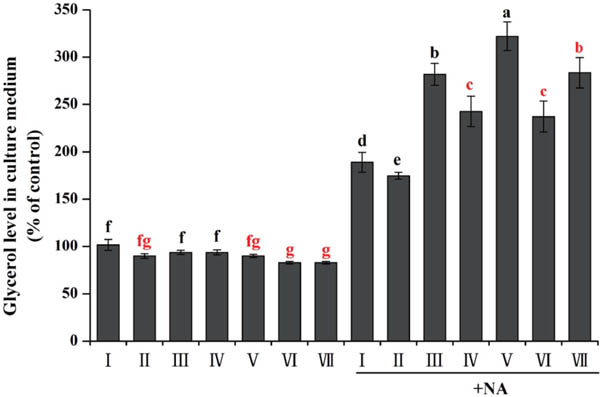
Effect of PNP and KF combination on lipolysis in 3T3-L1 cells differentiation on 8th day.
I: control; II: 40 µg/mL KF; III: 160 µg/mL PNP; IV: 20 µg/mL KF+80 µg/mL PNP; V: 20 µg/mL KF+160 µg/mL PNP; VI: 40 µg/mL KF+80 µg/mL PNP; VII: 40 µg/mL KF+160 µg/mL PNP.
All data are presented as the mean ± SE of six independent experiments. Means within the same column but not sharing the same superscript letter are significantly different (P < 0.05)
Effect of PNP and KF combination on secrete level of cytokines The effects of PNP and KF on 3T3-L1 cells secretion of cytokines were shown in Table 2. PNP and KF combination significantly decreased secretion of leptin, TNF-α and IL-6, and significantly increased the concentration of the adiponectin, when compared with control.
| Adiponectin | Leptin | TNF-α | IL-6 | |
|---|---|---|---|---|
| I | 397.96±4.92f | 426.34±4.31a | 87.54±0.33a | 140.65±1.92a |
| II | 439.41±3.92e | 351.22±3.52c | 69.30±0.62bcd | 100.66±1.16d |
| III | 517.88±4.44b | 396.3±4.32b | 80.08±0.81ab | 124.22±1.41b |
| IV | 436.82±2.33e | 409.96±2.61b | 79.29±0.66ab | 122.03±1.91b |
| V | 504.77±5.67c | 336.78±3.18cd | 73.41±0.36bc | 115.93±0.75c |
| VI | 487.44±1.57d | 328.97±6.45d | 71.04±1.02cd | 89.01±1.39e |
| VII | 548.66±2.73a | 269.47±5.52e | 62.79±1.23d | 70.05±0.77f |
All data are presented as the mean ± SE of six independent experiments. Means within the same column but not sharing the same superscript letter are significantly different (P < 0.05).
I: control; II: 40 µg/mL KF; III: 160 µg/mL PNP; IV: 20 µg/mL KF+80 µg/mL PNP; V: 20 µg/mL KF+160 µg/mL PNP; VI: 40 µg/mL KF+80 µg/mL PNP; VII: 40 µg/mL KF+160 µg/mL PNP.
Effect of PNP and KF combination on genes expression level mRNA expression levels of transcription factor results were summarized in Fig. 4A. Gene expression of PPARγ2, C/EBPα and SREBP1c were significantly down-regulated by PNP and KF combination in 3T3-L1 cells.
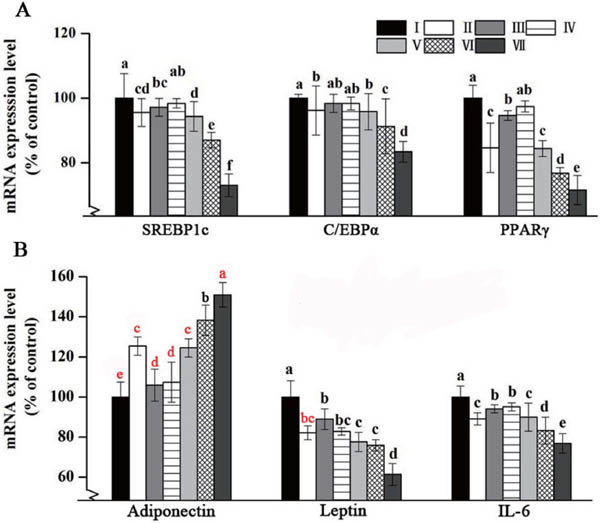
Effect of PNP and KF combination on gene expression level by RT-qPCR.
(A) Transcriptional factors gene expression level in cell differentiation on 8th day. (B) Cytokine gene expression level in cell differentiation on 8th day.
I: control; II: 40 µg/mL KF; III: 160 µg/mL PNP; IV: 20 µg/mL KF+80 µg/mL PNP; V: 20 µg/mL KF+160 µg/mL PNP; VI: 40 µg/mL KF+80 µg/mL PNP; VII: 40 µg/mL KF+160 µg/mL PNP.
All data are presented as the mean ± SE of six independent experiments. Means within the same column but not sharing the same superscript letter are significantly different (P < 0.05)
mRNA expression levels of cytokines were shown in Fig. 4B. PNP and KF combination significantly down-regulated mRNA expression of IL-6 and leptin, while adiponectin mRNA expression was significantly increased in 3T3-L1 cells, when compared with control.
Obesity was reported to lead to the development and progress of adipose tissue and adipocytes (Lefterova and Lazar, 2009). It was noted that the reduction of adiposity linked with the inhibition of adipogenesis along with the decrease lipid content of adipocytes (Kim et al., 2012). As a result, adipocytes differentiation and intracellular TG accumulations were closely associated with the development of obesity. In this study, the results revealed that PNP and KF combination remarkably suppressed fat accumulation and promoted lipolysis in 3T3-L1 adipocytes. Compared with control group, KF treatment alone significantly inhibited fat accumulation and PNP treatment alone increased lipolysis. Besides, their individual treatments were less effective than their combination. The research also suggested that PNP and KF presented potential anti-obesity effects through theirs common effects on inhibition of fat accumulation.
In recent years, the study showed that the increase of fat accumulation was the result of adipogenic process regulated by a complex network of transcription factors, PPARγ, C/EBPα and SREBP-1, which played major role in regulating the process of terminal differentiation (Cristancho and Lazar, 2011). PPARγ containing two splicing variants, ubiquitously expressed PPARγ1 and adipocyte-specific PPARγ2 (Takenaka et al., 2013), it was a master regulator of adipogenesis and was induced by C/EBPβ, and C/EBPα plays a role in maintaining the expression of PPARγ during adipogenesis (Ishihara et al., 2018). SREBP-1c could control the production of endogenous ligand (s) for PPARγ, enhance the activity of PPARγ, and coordinate the actions of these adipogenic factors (Kim et al., 1998). In this study, PNP and KF combination suppressed PPARγ2 expression in the early stage of adipogenesis, meanwhile affected the expressions of C/EBPα and SREBP1c, suggesting the suppression of PPARγ by the combination was dependent of C/EBP signaling and the suppression of SREBP-1c expressions was generally observed in adipocyte differentiation. Over-expression of PPARγ2 and C/EBPα in adipose tissues resulted in the acceleration of dipogenesis (Tontonoz et al., 1994). It has been reported that significantly down-regulation the expression of PPARγ, C/EBPα, and SREBP-1c in 3T3-L1 cells, could inhibit adipogenesis (Sung and Lee, 2015). However, up-regulation the mRNA expression of PPARγ, C/EBPα increased the fat accumulation in 3T3-L1 cells (Shen et al., 2017). As a consequence, it was possible that down-regulation the expression of PPARγ, C/EBPα and SREBP-1c inhibited adipogenesis and fat accumulation. The present results showed that PNP and KF combination suppressesed adipocyte differentiation through inhibition of the expression of PPARγ2, C/EBPα and SREBP-1c in the early stages of adipocyte differentiation, at the same time, the combination dose-dependently regulated the transcription factors. These results revealed that PNP and KF combination decreased fat accumulation might be ascribed to the molecular mechanism of down-regulation the gene expression level of PPARγ, C/EBPα and SREBP-1c.
Pine needle water extract suppressed differentiation of 3T3-L1 cells, in part by down-regulating mRNA expression of PPARγ (Jeon and Kim, 2006). Some reported show that polysaccharide can inhibited the expression of both C/EBPα and PPARγ (Kim et al., 2010), and show stimulated lipolysis activity to a certain extent (Kanagasabapathy et al., 2014). Therefore, PNP as active polysaccharide belong to pine needle extract, may inhibited fat accumulation by inhibited the expression of both C/EBPα and PPARγ, and promoted lipolysis. In fact, PNP alone in this experiment remarkable stimulated lipolysis, certain inhibited the expression of PPARγ but not significant inhibited fat accumulation. Furthermore, Puerarin as a principal component in KF, increased gene expression level of PPARγ2 to inhibited fat accumulation (Zheng et al., 2015). Our research indicated that co-treatment with PNP and KF significantly decreased mRNA expression of PPARγ2, their effect of combined treatment was much better than effect of individual treatment. Thus PNP and KF combination may enhance fat metabolism though decrease mRNA expression of PPARγ2 to inhibited fat accumulation, and PNP contributing to stimulated lipolysis. As a result, combination of PNP and KF exhibited better anti-obesity effect.
The expression profiles of adipokines and cytokines, such as adiponectin, leptin, TNF-α and IL-6 were altered by obesity (Khan et al., 2013). Adiponectin presented in plasma at a very high concentration, and adiponectin levels were negatively correlated with visceral adiposity (Yuji, 2010). A research showed that adiponectin gene expression were significantly suppressed by IL-6 in 3T3-L1 adipocytes (Fasshauer et al., 2003). In this work, significant decreases of IL-6 levels by PNP and KF combination led to increase of the adiponectin gene expression. Leptin significantly reduced fat accumulation and inhibited maturation of preadipocytes (Ambati et al., 2007). However, TNF-α treatment of both 3T3-L1 adipocytes and mice all caused increase in plasma leptin levels, TNF-α could directly regulate the release of leptin on adipocytes (Kirchgessner et al., 1997). Furthermore, enhancement of plasma leptin level was correlated to increase of the expression of C/EBPα (Kang et al., 2014), C/EBPα-mediated transactivation of the leptin promoter (Hollenberg et al., 1997). Our data showed that PNP and KF combination inhibited mRNA expression of C/EBPα, and decreased secretion of TNF-α, thereby leptin gene expression and secretion were reduced. PNP and KF combination treatment regulated gene expression of cytokines that resulted in increasing adiponectin secretion, decreasing secretion of leptin and IL-6. It was probable that TNF-α and transcription factor might participated in regulating gene expression of cytokines. These results suggested that PNP and KF exerted synergy regulation of the expression of cytokines in the process of adipocyte cell differentiation.
In this study, the anti-obesity properties of PNP and KF combination in 3T3-L1 cells was evaluated. The results indicated that PNP and KF combination could be useful in the treatment of obesity due to inhibition of fat accumulation and promotion of lipodieresis. PNP and KF combination reduced fat accumulation during the early stage of adipocyte differentiation through inhibition of the gene expression of metabolism-related transcriptional factors PPARγ2, C/EBPα and SREBP-1c to some extent, which in turn regulated the gene expression and secretion of cytokines, such as TNF-α, IL-6, adiponectin and leptin. In addition, how these factors affected in vivo are future prospective studies and necessary to be investigated further.
Acknowledgments The present study was kindly supported by National Natural Science Foundation of China (No. 81760157), Science Technology Agency of Jiangxi Province, China (No. 2016BBF60086) and Modern Agricultural Scientific Research Innovation Special Fund Projects of Jiangxi Province, China (No. JXXTCX2017003-03).