2018 年 24 巻 6 号 p. 1021-1027
2018 年 24 巻 6 号 p. 1021-1027
The fresh-cut iceberg lettuce inoculated with Listeria Monocytogenes (3 log CFU/mL) was packaged in modified atmospheres (10% CO2/10% O2, 30% CO2/10% O2, 50% CO2/10% O2) and then stored at 4°C, 8°C, 16°C, 24°C and 32°C separately. Modified Gompertz model was selected to estimate the growth rate and lag time. Results showed that the growth of L. monocytogens decreased with the increase of concentration of CO2 and revised Gompertz equation fitted the growth of L. monocytogens at different test conditions. Root Square model was chosen as a secondary model to study the effects. Mathematical evaluation was also conducted with the bias factor (Bf) and accuracy factor (Af) and residual analysis. Results showed that predictive value of the model is more accurate and reliable in level of α=0.1 and the value of Bf all is in 0.7–1.05, while the value of Af is acceptable in general.
Listeria monocytogenes is an important foodborne pathogen that causes listeriosis and other diseases including sepsis and meningitis, which has a high mortality rate (Mead et al. 1999; Szabo et al. 2003; Carrasco et al. 2008). As a facultative anaerobe, L. monocytogens is able to grow at temperatures ranging from −1.5 to 45°C, pH from 4.39 to 9.40, and high osmotic conditions (Te and Zwietering, 1999). Hence, L. monocytogens has raised serious concerns in minimally processed foods. The contamination of L. monocytogens in raw or processed vegetables has been frequently reported (Samelis et al. 2001; Kramarenko et al. 2016). One possible reason is the current industrial processing practice of vegetables, such as the sanitized washing, which does not provide thoroughly cleaning of the pathogen (Kramarenko et al. 2016).
Modified atmosphere packaging (MAP) is a popular strategy to inhibit the growth of microorganisms in foods to maintain their quality with extended shelf life (Xu et al. 2017). The applications of MAP have been used to protect raw and cooked meat, fish, fresh fruit and vegetables (Liamniniter et al. 2018; Parks et al. 2012; Han et al. 2010). O2, CO2 and N2 are generally used in MAP, of which CO2 is the major anti-microbial component. Low levels of O2 with elevated CO2 concentrations are typically applied to suppress respiration rate, retard ripening and senescence, and associated with quality loss of fruits and vegetables (Resconi et al. 2012). Fresh-cut vegetable are easily infected by L. monocytogens during MAP processing. Although the growth of L. monocytogens on lettuce has been previously reported (Kramarenko et al. 2016; Couvert et al. 2017; Koseki et al. 2005), the growth of this pathogen on fresh-cut iceberg lettuce under the MAP that is initially flushed with different concentrations of CO2, O2 and N2 gases is absent from the literature. There is an urgent need to study the growth of L. monocytogens on fresh-cut lettuce processed by MAP to avoid food poisoning.
Numerous bacterial growth modes have been developed to predict the behavior of microorganisms. Highly sophisticated models are available, including Baranyi, Logistic and modified Gompertz models (Luo et al. 2015; Sheen et al. 2011). Large amount of work has been done in relation to growth models of L. Monocytogenes. Farber et al. (1996) investigated the effects of pH (5.5–6.5), temperature (4–10°C) and CO2 (10–90%) on the growth of L. monocytogens in brain heart infusion broth. Fernándcz et al. (1997) used Baranyi and Roberts model to fit the growth curves and compared predictions of growth from the model. However, most of those models were built on nutrient broth experiments in laboratories, resulting in the overestimation of the growth of L. monocytogens in real foods and making those models not practically useful. To our knowledge, a model that is developed for predicting the effect of CO2 on the growth of L. monocytogens in fresh-cut iceberg lettuce is not reported. The aims of this work were to study the effect of a wide range of CO2 concentrations (10%–50%) on the growth of L. monocytogens in fresh-cut iceberg lettuce held at temperatures ranging from 4 to 32°C under MAP; and to develop a predictive model for the growth of this pathogen in MAP fresh-cut lettuce.
Strain Listeria monocytogenes CICC 21662 was obtained from the China Center of Industrial Cultural Collection (Beijing, China). A freeze-dried culture of this strain was thawed and streaked onto agar (PALCAM agar base with selective supplements) and the plates were incubated at 37°C for 48 h. Single colony from the PALCAM plate was transferred to 10 mL tryptic soy broth (TSB) tubes and incubated at 37°C for 4 h, and then transferred to another tube of fresh TSB and incubated at 37°C for 18 h, to a level about 108 CFU/mL. The culture was diluted and dispersed in sterile saline solution to obtain the final concentration of 103 CFU/mL as the concentration for inoculum.
Sample preparation Freshly unprocessed iceberg lettuce was obtained from a local farm and transported to the lab within 1 h. The lettuce heads were processed at 7°C in a clean room. The inner leaves were individually washed using tap water twice and drained on absorbent paper. The leaves were cut into pieces (5×5 cm) by knifes. Both sides of lettuce were sterilized under UV for 20 min to receive a dose of 1.5 kJ/m2 respectively to eliminate the background bacteria according to Seong et al. (2017). After sterilization, each lettuce sample (10 g) was placed into a sterilized plastic box for inoculation treatment.
Inoculation and storage of iceberg lettuce in MAP The sample was inoculated with 1 mL suspension of L. Monocytogenes, and then shaken gently for 30 times. Once inoculated, the box was aerated using a composite modified atmosphere packaging machine. The proportions of CO2, O2 and N2 in the package headspace were as the following: MA1 (10% CO2, 10% O2, 80% N2), MA2 (30% CO2, 10% O2, 60% N2), and MA3 (50% CO2, 10% O2, 40% N2). Inoculated lettuce samples sealed in different MAP were stored at 4, 8, 16, 24 and 32°C, and samples were collected at different time intervals to be analyzed. The gases were created and adjusted every 8 h to the desired concentration by a flushing of CO2, O2 or N2 into the containers, using the device previously described by Carlin et al. (1996). The time intervals between sampling varied depending on the temperature of storage and ended once the microorganisms reached stationary phase. Triplicate growth curves were performed. The samples were added with 90 mL sterile saline and homogenized for 2 min. For an enumeration, 0.1 mL of such dilute was spread on the surface of a PALCAM agar plate, and the plate was incubated at 37°C for 24 h.
Establishment of the primary and secondary models Growth curves of L. monocytogens for each combination of the temperature and CO2 concentration were built separately by fitting data to the Gompertz model modified by Zwietering et al. (1993) using SigmaPlot 10.0. Each combination was replicated three times to estimate two curve parameters: growth rate (µmax) and lag time (λ).
 |
where t is time (h); Nt is the number of cells at time t (CFU /g); N0 and Nmax are the initial and final number of cells (CFU / g), respectively; µmax is growth rate (h−1) and λ is lag time (h).
The secondary growth model described the dependence of the primary model parameters on environmental factors. The effect of temperature on microbial growth kinetics was usually described by square root model (Ratowsky et al. 1982), which demonstrated a linear relationship between temperature and the growth rate or the reciprocal of lag time. The model was as follows:
 |
 |
where µmax is the growth rate (h−1); λ is lag time (h); T is temperature (°C); Tmin is the conceptual minimum temperature for microbial growth; bµmax and bλ are the slopes of the regression coefficients. The parameters of b and Tmin were estimated by searching values of T and µmax using the Eq.1 before the effect of temperature on µmax was obtained.
After amended by Devlieghere et al. (2001), the factor of CO2 concentration was taken into consideration and added to the root square model as follows:
 |
 |
where bµ max and bλ is constant, Tmin is the respective estimated theoretical minimum temperature (°C), CO2 max is the respective estimated theoretical maximum CO2 concentration (mg/L), CO2 is the concentration of dissolved CO2 (mg/L), refers to the concentration of CO2 without metabolic significance.
Validation of models In order to evaluate the goodness-of-fit of the model, the bias factor (Bf) and accuracy factor (Af) proposed by Ross et al. (1996) and Mellefont et al. (2003) were adopted and calculated by the following equations:
 |
 |
where pred was the predicted value of growth parameters using root square model; obs was the observed values of growth models; n is the number of trials.
According to Ross (1999), Bf is used to evaluate the extent of under-or over-prediction by the model of the µmax and lag time observed, when Bf is above 1, it indicates that the predicted µmax is lower than the observed value, which means safe in practice. In model performance evaluations, if the values of Bf are between 0.9 and 1.05, the model can be considered good; if the values are between 0.7–0.9 or 1.05–1.15, the model can be considered acceptable. Af was calculated for providing an indication of the average of estimates, the average estimates tend to be less accurate with the increase of Af. (Lebert et al. 2000).
Although the Bf and Af can be used to validate the models, they can't describe the systematic deviation. Therefore, Baranyi et al. (2000) suggested using the residual analysis to describe the systematic deviation, in which the residual is the difference between the predicted value and the observed value. In the current study, the residual analysis was also conducted to assess the performance of the models.
Data analysis Data were obtained from two independent experiments and analyzed by ANOVA using SAS (Version 8.2; SAS Institute, Cary, NC, USA). The P value ≤ 0.05 was considered statistically significant.
Estimation of growth parameters and primary predictive model The growth of L. monocytogens in fresh-cut lettuce at different CO2 concentrations from 4°C to 32°C is shown in Fig. 1. The growth curves were predicted using modified Gompertz equation. The modified Gompertz equation works well for the prediction of L. monocytogens growth in fresh-cut lettuce under tested conditions. Table 1 shows the two parameters of µmax and λ of the growth curve in different conditions. The µmax of L. monocytogens was lower and λ was longer at the same temperature with the increasing of CO2 concentration (from 10% to 50%). At 32°C, when the concentration of CO2 increased from 10% to 50%, the µmax of L. monocytogens decreased from 0.341 (h−1) to 0.195 (h−1), while the λ increased from 3.792 h to 9.688 h. This indicated that CO2 significantly inhibited to the growth of L. Monocytogenes.

Growth curves of L. monocytogens on fresh-cut lettuce at a constant temperatur. (4, 8, 16, 24 or 32°C) under modified atmosphere packaging using modified Gompertz model.
| Temperature (°C) | Concentrations of CO2 (%) | Gompertz equation | |
|---|---|---|---|
| µmax (h−1) | λ (h) | ||
| 4 | 10 | 0.024 | 101.557 |
| 4 | 30 | 0.018 | 125.846 |
| 4 | 50 | 0.015 | 140.427 |
| 8 | 10 | 0.038 | 60.430 |
| 8 | 30 | 0.026 | 68.781 |
| 8 | 50 | 0.022 | 83.883 |
| 16 | 10 | 0.070 | 15.516 |
| 16 | 30 | 0.055 | 19.938 |
| 16 | 50 | 0.038 | 25.523 |
| 24 | 10 | 0.165 | 6.288 |
| 24 | 30 | 0.135 | 11.764 |
| 24 | 50 | 0.095 | 16.448 |
| 32 | 10 | 0.341 | 3.792 |
| 32 | 30 | 0.240 | 5.525 |
| 32 | 50 | 0.195 | 9.688 |
µmax, growth rate, λ, lag time.
Establishment of the secondary predictive model The µmax obtained using the primary model and the corresponding temperatures were substituted into Eq. 2 and 3, respectively. Fmincon function in Matlab7.1 was applied using iterative method to fit the value of bµ max, bλ and CO2 max. Equations are as follows:
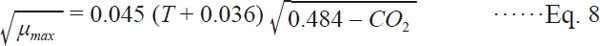 |
 |
Model validation and verification The model was verified at random time points under conditions of each group. Table 2 illustrates the mathematical validations of the secondary model of CO2 inhibition of L. Monocytogenes. As it can be seen, the Bf ranged from 0.766 to 1.047. According to the critical values of Bf proposed by Mellefont et al. (2003), all Bf values obtained in this study fall in the range of acceptable level. For the data set at 10% CO2 concentration, Bf values are all in good level, expect at 32°C with Bf value of 0.833 (considered acceptable). The Af values are all higher than 1, and at 8°C with 10% CO2 concentration, the value of Af reached to 1.311, other values are all within the range of 1.0–1.3. Ross et al. (2000) reported that an acceptable Af could be determined by considering the effect of the number of environmental parameters in a kinetic model. Therefore, the acceptable performance that might be expected from the Kinetic model encomp assing the effects of temperature and CO2 concentration, is about 30% or an Af of 1.3. Therefore, for the value of Af, the model is accepted in general.
| Temperature (°C) | CO2 concentrations (%) | Trial No. | Bf | Af |
|---|---|---|---|---|
| 4 | 10 | 6 | 0.928 | 1.222 |
| 4 | 30 | 6 | 1.035 | 1.186 |
| 4 | 50 | 6 | 0.862 | 1.23 |
| 8 | 10 | 6 | 0.949 | 1.311 |
| 8 | 30 | 6 | 0.766 | 1.127 |
| 8 | 50 | 6 | 0.973 | 1.279 |
| 16 | 10 | 6 | 0.956 | 1.024 |
| 16 | 30 | 6 | 0.782 | 1.178 |
| 16 | 50 | 6 | 0.886 | 1.232 |
| 24 | 10 | 6 | 0.923 | 1.185 |
| 24 | 30 | 6 | 0.991 | 1.217 |
| 24 | 50 | 6 | 0.876 | 1.155 |
| 32 | 10 | 6 | 0.833 | 1.288 |
| 32 | 30 | 6 | 0.915 | 1.132 |
| 32 | 50 | 6 | 1.047 | 1.264 |
Trial no., random trial mumbers for residum analysis, Bf, bias factor, Af, accuracy factor
To evaluate the model more accurately, random verification experiments were conducted six times and the results are shown in Fig. 2. It demonstrated that the absolute values between the predicted and observed values in different conditions are all < 0.1, that means the predictive value of the model is liable at the level of α = 0.1 in the 95% confidence interval.

The residual analysis of model. Triangle represents the cells of L. monocytogens in the condition of MA1 at random time points. Dot represents the cells of L. monocytogens in the condition of MA2 at random time points. Square represents the cells of L. monocytogens in the condition of MA3 at random time points.
The modified root square model as the secondary model used in the current study performed well for the growth of L monocytogenes in the MAP lettuce with different temperature and CO2 combinations.
Generally, many studies have found that CO2 (≥ 20%) reduce growth rate and extend lag time in the in vitro test. (Resconiet et al. 2012; Delieghere and Debevere 2000). In this study, a predictive model for relationship between CO2 and L. monocytogens in fresh cut lettuce was developed. Consistent with the above researches, our results confirmed that the growth of L. monocytogens was inhibited with increasing concentrations of CO2. Also, the µmax of L. monocytogens was lower and λ was higher with the increasing of CO2.
Predictive models offer application potential in food microbiology. In recent years, despite the increasing association of vegetables with foodborne disease outbreaks (Ross, 2000), comparing with meat and dairy products (Mcmeekin, 2007; Poirazi et al. 2007; Lindgvist et al. 2011), few studies have been conducted on predicting the growth of pathogens in fresh vegetables (Couvert et al. 2017). Therefore, there is a need for more data on the growth of foodborne pathogens in these products. Zwietering et al. (1996) compared Gompertz, Richards, Stannard, Schnute, and Logistic models that describe the s-type growth curve and concluded that only corrected Gompertz equation fits the growth pattern of L. monocytogens (Farber et al. 1996). In this study, growth curves for each temperature and CO2 concentration were built separately by fitting data to the Gompertz model, and results showed a high correlation coefficient at all temperatures (R2>0.95), which proved that Gompertz function, as a dynamic model, could deal with time varying environmental conditions for prediction of L. Monocytogenes.
The secondary model applied is Bělehrádek or Ratkowsky Square Root model. Square root model has been applied to describe how single factor influence the growth of microorganisms. However, this model has limitations in describing how do multi-factors influence the growth of microorganisms (Devlieghere et al. 2001). In related studies, it can be replaced using responsive surface methodology, artificial neural network model or correct square root model. But square root model is commonly used for its single parameter and simple use, and can predict the growth condition well under univariate. In this study, two factors of temperature and CO2 concentration were taken into account, and Eqs. (8) and (9) were finally got to describe the effect of temperature and CO2 concentration on growth of L. Monocytogenes.
Results showed that the growth of L. monocytogens was inhibited along with the increase of concentration of CO2, especially when the CO2 concentration was 50%, the growth of L. monocytogens was significantly inhibited. That is to say, CO2 has significant inhibition of the growth of L. monocytogens on fresh-cut lettuce and revised Gompertz equation has a good statistic fit to the growth of Listeria monocytogenes at different test conditions with a high correlation coefficient (R2>0.95). And Root Square model was chosen as a secondary model to study the effects of temperature and CO2 concentration on growth kinetic parameters of Listeria monocytogenes in the primary model. Mathematical evaluation was also conducted with the Bf and Af and residual analysis. Results showed that predictive value of the model is more accurate, and reliable in a level of α=0.1, and the value of Bf all is in 0.7–1.05, while the value of Af is accepted in general, namely the model can be accepted. Therefore, the model can predict the growth of Listeria monocytogenes in fresh-cut iceberg lettuce successfully.
Acknowledgements This study was supported by the project 2017YFD0400904-2.