2018 年 24 巻 6 号 p. 1029-1038
2018 年 24 巻 6 号 p. 1029-1038
Melanin extracted from sunflower testae was isolated, purified and the physicochemical properties, antioxidant activities, metal ion removal activity and anti-radiation were investigated. The physical and chemical characterization showed that it was acid-resistant, alkali soluble and insoluble in most of the organic solvents and distilled water and the similar redox properties to synthetic melanin from sigma. The melanin was precipitated on treatment with FeCl3, ammoniacal AgNO3, potassium ferricyanide and was bleached in the presence of oxidants and reductants. It was also confirmed as one of melanins based on the Ultraviolet visibl. (UV-vis) absorption spectroscopy, Fourier transform infrared spectrometry (FT-IR), Electron paramagnetic resonance (EPR) spectroscopy, and Scanning electron microscopy (SEM) spectroscopic techniques apart from physiochemical analysis. Elemental composition analysis revealed that the melanin was pheomelanin or allomelanins. The melanin also showed the antioxidant potential observed in the in vitro evaluation of diphenyl picryl hydrazinyl radical scavenging activity, superoxide-scavenging activity, hydroxyl-scavenging activity, reducing power, metal ion removal activity and anti-radiation activity. The activities examined indicated that melanin from sunflower testae could be considered as a novel potential antioxidant, heavy metal adsorbent, and UV protection agent. The results suggested that the melanin could potentially be used as a natural antioxidant in the food, cosmetic and other industries.
Pollution had occurred globally through different ways such as industry, agriculture and light radiation. Many heavy metals had been discharged into the environment as industrial wastes, causing serious soil and water pollution (Lin et al., 2002). About 2.4 million tons of Pb, Cu, As, Cd, Cr, Hg, Ni, Se, V, and Zn had been generated annually by fuel and power industries (Sajjan et al., 2013). Pb2+, Cu2+, Fe2+, and Cr3+ were the most common in all the metal ions and tended to accumulate in living organisms, causing numerous diseases and disorders (Inglezakis et al., 2003). The adsorption, which was a potential alternative technique for removing heavy metals like ion exchange, precipitation and electro dialysis, was one of the most promising technologies for the removal of heavy metals from natural and industrial waste waters. Apart from heavy metals pollution, excessive UV radiation, which was brought about air pollution and ozone layer destroyed, could lead to skin ambustion or cancer, suppression of immune system and cataracts (Ye et al., 2014). Studies found that plant extracts of gardenia fruit, licorice rhizome and rhizome coptidis had anti-ultraviolet activities (Kato et al., 2012). Therefore, the exploring and developing of natural bio-adsorbent and anti-radiation components would keep an important application value.
Melanin is an irregular red, brown, dark brown to black polymer of macromolecules formed by oxidative polymerization of phenol and/or indolic compounds, which widely exists in animals, plants and microorganisms. Melanin, derived from such biological sources as Sepia officinalis, black-bone silky fowl, Osmanthus fragrans seeds, tea leaves, Hypoxylon archeri, Auricularia auricular, Tuber mehnosporum, Ophiocordyceps sinensis, Ommastrephes bartrami, Streptomyces lusitanus, Aspergillus fumigatus, Lachnum singerianum, Paecilomyces variotii, Aspergillus carbonarius, and so on (Zhang et al., 2015; Guo et al., 2014), show similar physical and chemical properties. These physico-chemical properties include strong light absorbance, unusual solubility and remarkable redox properties, and also has a number of healthful functions and a broad biological activities, such as antioxidant, free radical-scavenging, antimicrobial activity, antitumor activity, antivenin activity, anti-HIV activity, liver protecting activity, immunomodulatory activity, radio protective and so on (Zou et al., 2010; Sun et al., 2016a; Sun et al., 2016b). These functions and biological activities promise natural melanin with great development potential as a healthful food and cosmetic colorant, even pharmacology, medicines and others.
Sunflower seeds are divided into two types of oil seeds and snack seeds. Both seeds have rich in husk, the husk is more than 40% in oil seeds and about 20% in snack seeds. Moreover, sunflower testae is abundant in melanin and melanin is increasingly popular in natural cosmetic, even in medicines, agriculture, bioelectronics, heavy metals extraction and so on. However, sunflower testae have been usually burned directly or deep buried, caused severely air pollution, a lot of land taken and resource wasted. Up to now, there are seldom reports on the investigations of melanin extracted from sunflower testae. Furthermore, it is hard to get single component of this melanin to analyze its structure. In the present study, the melanin extracted from sunflower testae was determined and analyzed. The structure and biological activities of melanin from sunflower testae has then been investigated.
Materials and reagents Sunflower seed was purchased from a local market in Changzhi City (Shanxi Province, China), peeled, pulverized and sifted through a 60-mesh sieve. The powder (moisture content 12–15%) was stored in dark bags to be kept from moisture and light. 2,2-Diphenyl-1-picrylhydradyl (DPPH) and synthetic melanin/standard melanin (3,4-Dihydroxyphenylalanine) were purchased from Sigma Chemicals Co. (St. Louis, USA). All the other chemicals and reagents used in the experiment were of analytical grade.
Melanin extraction and purification Briefly, 10 g of sunflower testae was boiled with 100 mL deionized water for 15 min at 100°C, cooled to room temperature, centrifuged at 4°C 10000 rpm for 10 min, discarded the supernatant and took the residue. And then the residue was homogenized in 100 mL 1 mol/L NaOH for 5 min using an ultrasonic processor (Sonic VCX130, USA) equipped with a 13 mm microtip, extracted at 75°C for 3 h under reflux in an atmosphere of nitrogen. Crude melanin extraction were first adjusted to pH 2.0 with 3 mol/L HCl to precipitate melanin, followed by centrifugation (Beckman Avanti J-26) at 10,000 rpm for 10 min for collecting the pellet. Crude melanin was hydrolyzed using 6 mol/L HCl at 100°C for 2 h. Non-hydrolysable melanin collected by centrifugation (10,000 rpm, 20 min) was washed successively with chloroform, ethyl acetate, and ethanol, then dissolved in 1 mol/L NaOH. The pH of melanin-sodium hydroxide solution was adjusted to 2.0 with 3 mol/L HCl. After centrifugation (10,000 rpm, 20 min), the pellet was washed with deionized water until pH was 7 at the supernatant. The purified melanin was collected and lyophilized. Further purification was carried out according to the method with slight modification (Zou et al., 2015). The melanin was dissolved in 100 mL of 0.1 mol/L phosphate buffer (pH 8.0), then loaded onto a Sephadex G-50 column (1.6×60 cm) equilibrated using 0.1 mol/L phosphate buffer (pH 8.0). The flow rate was 1 mL/min. The fractions were collected with each tube containing 1 mL. After washing the column with the same buffer, the different fractions were obtained. The absorptions of fraction was measured at 400 nm, the same fractions were combined, dialyzed, lyophilized and stored at −20°C.
Preliminary physico-chemical properties assay The physico-chemical properties of melanin was measured according to the method (Wang et al., 2006; Fava et al., 1993; Pan et al., 2009; Sava et al., 2001a; Sava et al., 2001b). The solubility of the melanin in distilled deionized water, 0.1 mol/L hydrochloric acid (HCl), 0.1 mol/L sodium hydroxide (NaOH), 0.1 mol/L sodium bicarbonate (NaHCO3), 0.1 mol/L sodium carbonate (Na2CO3), 0.2% ammonia, ethanol, methanol, ethyl acetate, ether, petroleum ether, butanol, acetone, chloroform, benzene, and phenol were determined. Reactions with oxidizing agents such as sodium hypochlorite (NaOCl), hydrogen peroxide (H2O2), potassium permanganate (KMnO4), and potassium dichromate (K2Cr2O7) were determined. Reducing agents such as H2S and 5% sodium hydrosulfite (Na2S2O4) were also tested for reaction with the melanin. The melanin was also precipitated with 1% ferric chloride (FeCl3), ammoniacal silver nitrite, and potassium ferricyanide (K3[Fe(CN)6]).
UV-Vis spectrum analysis After the preparation of 50 mg/L melanin solution, the absorption properties of melanin were analyzed with UV-Vis spectroscopy (Thermo Scientific Evolution 300,USA), which was recorded in the wavelength range of 190–800 nm using 0.1 mol/L NaOH solution as the reference.
FTIR analysis For FTIR studies, melanin-KBr pellets were prepared as follows: firstly, KBr was homogenized in an agate mortar and weighed to obtain about 200 mg; secondly, melanin was homogenized in an agate mortar and weighed to about 10 mg; next, weighed KBr and lactase were mixed well in an agate mortar; finally, the melanin-KBr mixture was made into a tablet with a Specachydraulic pellet press. Melanin-KBr pellets were analyzed with FTIR at wave numbers 4000–500 cm−1. A Nicolet AVATAR360 instrument was utilized in this study. The resolution of the FTIR instrument was 4.0 cm−1, optical path difference (OPD) velocity 0.20 cm. s−1, and the data collection interval 1.0 s. The actual spectra were measured as absorbance ratio. Background spectra were measured from clean KBr pellet containing no sample material.
EPR Spectroscopy The electron paramagnetic resonance (EPR) spectrum of the melanin extracted from sunflower testae was recorded in a solid state at room temperature in quartz tubes on a JES-FA200 X-band EPR spectrometer (JEOL, Tokyo, Japan) operating at 100 kHz modulation frequency and the g-value of the sample was measured at 25°C.
Element composition Percentages contents of C, H, N, O and S in the melanin extracted from sunflower testae were determined by elemental analyzer (Elementar Vario EL cube, German).
Morphology analysis Melanin extracted from sunflower testae was scanned and imaged for different amplified times using FEI Quanla 200 scanning electron microscope with a working voltage of 15 kV and a working distance of 17.0 mm.
Bioactivity assay
DPPH radical-scavenging activity assay Antioxidant activity of melanin extracted from sunflower testae was ex amin ed b y the metho d w ith s ome mo dif ication s (Manivasagan et al., 2013). Two milliliter sample at different concentrations was mixed with 2 mL absolute ethanol and 500 µL 0.02% DPPH in absolute ethanol. The mixture was shaken vigorously and left to stand in the dark for 30 min, and the resulting solution was measured at 517 nm using a UV-visible spectrophotometer.
 |
Melanin was sample, ethanol was used as control, and ascorbic acid and synthetic melanin (standard melanin from sigma) were used as standards.
Superoxide radical (O2−.)-scavenging activity assay The superoxide radical (O2−.) scavenging activity was determined according to the method with some modifications (Duan et al., 2007). The assay was based on the ability of antioxidant to inhibit formazan formation by scavenging the superoxide radical generated in riboflavin-light-nitrotetrazolium blue chloride (NBT) system. All solutions were prepared in 0.05 mol/L phosphate buffer (pH 7.8). Each 6.5 mL of reaction mixture contained 2 mL L-methionine (39 mmol/L), 2 mL NBT (225 µmol/L), 1 mL ethylenediaminetetraacetic acid disodium salt (EDTA-Na2, 0.6 mmol/L), 0.5 mL water or melanin at different concentration, as well 1 mL riboflavin (12 µmol/L). The reaction solution contained sample was illuminated for 15 min at 25°C. The absorbance was detected at 560 nm. The reaction mixture, without sample, was used as a control and kept at the dark. The scavenging activity was calculated as follows:
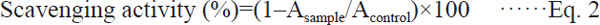 |
Hydroxyl radical scavenging activity assay The effect of antioxidant on hydroxyl radical was studied by method with some modifications (Jin et al., 1996). (a) 1 mL 0.75 mol/L 1,10-Phenanthroline was mixed with 0.2 mmol/L phosphate buffer (pH 7.4, 2 mL) and 1 mL double distilled water, shook fully, added to 1 mL 0.75 mmol/L ferrous sulfate (FeSO4), then shaken, and added to 1 mL 0.01% hydrogen peroxide (H2O2). The mixture was incubated at 37°C for 60 min. The absorbance was measured at 536 nm and called Af. (b) The method was same with (a), however, 1 mL double distilled water was used to replace 1 mL 0.01% H2O2 and the absorbance was called A0. (c) The method was same with (a), except that 1 mL sample was substituted for 1 mL double distilled water and the absorbance was called AX. (d) The method was same with (a), except that 1 mL sample was substituted for 1 mL H2O2 and the absorbance was called As. Hydroxyl radical scavenging activity was calculated according to the formula:
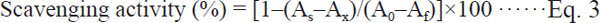 |
Reducing power determination The reducing power of melanin was determined according to the method of Hsieh et al (2000). Samples at different concentration were mixed with phosphate buffer (2.5 mL, 0.2 mol/L, pH 7.6) and K3[Fe(CN)6] (2.5 mL, 1%). The mixture was incubated at 50°C for 20 min. Trichloroacetic acid (2.5 mL, 10%) was added to the mixture, which was then centrifuged for 10 min. The supernatant (2.5 mL) was mixed with distilled water (2.5 mL) and FeCl3 (0.5 mL, 0.1%). The absorbance was measured at 700 nm.
Metal ion absorption Adsorption of melanin on metals ion were conducted at different pH at five initial concentrations of Pb2+, Cr3+ and Cu2+ according to the method with some modifications (Ruziwa et al., 2015). Fifty milligram melanin was weighed into a 100 mL erlenmeyer flask which 50 mL metal ion solution was added in. The mixture was shaken in an air bath with homothermal vibrator (SHZ-82, Changzhou, China) for 6 h at 120 rpm and at room temperature. The samples were filtered and the filtrate was analysed for Pb2+, Cr2+ and Cu2+ using an ICP-AES (OPTIMA 8000DV, PE, USA).
Anti-radiation assay for melanin in vitro The anti-radiation of melanin was determined by the method with some modification (Ye et al., 2014). Bacillus thuringiensis (Bt) suspensions cultured for 48 h were taken and centrifuged at 10000 rpm for 10 min, and then the cells were harvested. Melanin was added to sterile saline to achieve a concentration of 2% (small amount of aqueous ammonia was added to dissolve melanin and then removed by evaporation). The melanin solution was added to the harvested bacterial cells as dilution to achieve a concentration of about 109 cfu/L. Ten milliliter diluted cells suspensions were placed in sterile culture medium (9 cm) and exposed to the UV254nm light source for 0 s, 30 s, 1 min, 2 min, 5 min, 10 min and 20 min, and then spread on the dishes and incubated at 37°C for 3 d. The plate count method was used to determine the survival Bt (A), and then the survival curves of Bt were established using the radiation duration as the abscissa and log A as coordinates.
Statistical analysis The Data was expressed by means ± standard deviation of triple determinations after one-way analysis of variance (ANOVA). Differences were considered statistically significant when p < 0.05 and very statistically significant when p < 0.01. Results were processed by SPSS 17.0 (SPSS Inc.) and Origin 7.0 (OriginLab Corporation).
Preliminary characterization of the melanin The physico-chemical analysis of the melanin from sunflower testae was showed in Table 1. The melanin was black, and insoluble in water, 0.1 mol/L acid, ethanol, methanol, benzene, chloroform, ethyl acetate, ether, petroleum ether, butanol, and acetone. The melanin was soluble in 0.1 mol/L alkaline solution, such as 0.2% ammonia water (60°C, 0.30 mg/mL), 0.1 mol/L sodium hydroxide (60°C, 1.81 mg/mL), 0.1 mol/L sodium hydrogen carbonate (60°C, 0.57 mg/mL), 0.1 mol/L sodium carbonate (60°C, 1.35 mg/mL). The melanin was decolorized by oxidizing and reducing reagents such as NaOCl, KMnO4, K2Cr2O7, H2O2, H2S, and Na2S2O4. The melanin exhibited positive for polyphenols with FeCl3, producing a flocculent brown precipitate, and reduced ammonical silver nitrate. These properties were similar to those of the melanin extracted from black tea leaves, O. fragrans' seeds, Black-bone silky fowl and synthetic melanin (Wang et al., 2006; Save et al., 2011a, 2011b; Tu et al., 2009).
| Number | Assay | Result |
|---|---|---|
| 1 | Water | Insoluble |
| 2 | Acid | Insoluble |
| 3 | Solubility in organic solvents (ethanol, methanol, benzene, chloroform, ethyl acetate, ether, petroleum ether, butanol, acetone, etc) | Insoluble |
| 4 | Alkaline solution (ammonia water, sodium hydroxide, potassium hydroxide, sodium hydrogen carbonate, sodium carbonate, etc) | soluble |
| 5 | Color | Black |
| 6 | Precipitation in pH < 2 | Precipitated readily |
| 7 | Reaction with oxidizing agent (H2O2, NaOCl, KMnO4, K2Cr2O7) | Decolorized |
| 8 | Reaction for polyphenols with FeCl3 assay | Brown flocculent precipitate |
| 9 | Reaction with Na2S2O4 and potassium ferricyanide | Decolorized and turned brown with addition of potassium ferricyanide |
| 10 | Reaction with ammoniacal silver nitrate solution | Formed a grey-colored silver precipitate lining on the sides of the test tube |
| 11 | Reduction with H2S gas | Reduced |
| 12 | UV-visible absorption | Linear relationship between log absorbance and wavelength between 400 and 600nm |
UV-Visible spectral properties The ultraviolet-visible absorption spectrum of melanin extracted from sunflower testae was showed in Fig.1a. No absorption peak at 280 nm was observed, indicating that there were no impurities of proteins, lipids and other. Melanin extracted from sunflower testae showed strong optical absorbance in Ultraviolet region and decreased progressively as the wavelength increased, and the decrease in the absorption with increasing wavelength was almost linear. The plot of log absorbance versus wavelength in the case of melanin gave line with a negative slope (Fig. 1b) and the slope was −0.0030.

(a) UV-VIS absorption spectrum of the melanin from sunflower testae. (b) A plot of log optical density against wavelength.
Infrared spectroscopy The IR spectra of melanin from sunflower testae were very similar to those reported, though small differences did occur (Fig. 2). The FTIR spectrum of melanin extracted from sunflower testae revealed: (1) a strong, broad band at 3422.95 cm−1, attributed to stretching vibrations of −OH and −NH2 groups; (2) The peak at 2930.24 cm−1 which appeared as a weak absorption band was caused by stretching vibration of CH2, CH3 and NH neighbouring to quinone (Senesi et al., 1987; Huang et al., 2009; Kumar et al., 2011; Ye et al., 2012). (3) The strong band at 1706.24 cm−1 caused by the vibration of C=O of −COOH and 1613.81 cm−1 due to the vibration of aromatic C=C and COO-groups. (4) Absorption bands at 1268.76 cm−1 were due to CO groups of acid, ester, and phenol groups. (5)Absorption appeared at 1029.19 cm−1 assigned to C-N stretch. (6)wagging vibration peak of N-H bond at 799.75 cm−1, and 591.76 cm−1 weak bands below 700 cm−1 ascribed to alkene C-H substitution in the melanin (Olennikov et al., 2011). The major difference was seen in the wavelength of 2930.24 cm−1, where melanin from sunflower testae has a peak caused by CH2 and CH3, and also by NH group oscillation, whereas the synthetic melanin showed no peak.
FT-IR spectrum of melanin extracted from sunflower testae
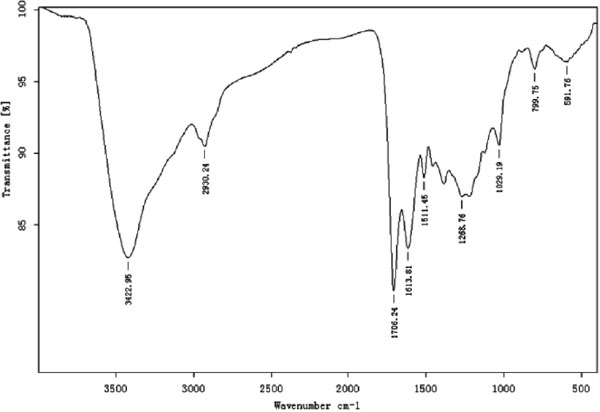
EPR spectroscopy Apart from UV-visible and FT-IR spectral study, EPR spectroscopic was determined to investigate the paramagnetic properties and free radicals present in the melanin from sunflower testaes. The features of melanin is the presence of a stable free radicals, which results in the characteristic electron paramagnetic resonance behavior (Fig. 3). The spectrum of the melanin extracted from sunflower testae was a slightly asymmetric singlet without hyperfine structure with about 13 Gauss width of the line between points of the maximum steepness and g = 2.030.
EPR spectrum of melanin extracted from sunflower testae
Elemental analysis Melanin extracted from the shell of sunflower snack seeds was composed of C, H, N, O, and S with the content percentages (%) of 61.125, 4.584, 1.740, 31.370 and 1.181, respectively. The content of elements were obviously different from those of other melanins reported (Sun et al., 2016b; Ye et al., 2014), indicating that content of elements in different melanins varied. According to the classification method of melanin, the results would mean that the melanin from sunflower testae should be classified as a phaeomelanin because of the presence of S. In addition, the C/H of melanin was relatively high (1.11), indicating the molecule contained relatively more aromatic structures (Latocha et al., 2000).
SEM images Fig. 4a–4b showed SEM images of melanin extracted from sunflower seeds husk. The appearance from the figure suggested that the material had an amorphous, porous deposit with no differentiable structures with an irregular surface, which was similar to that of the melanin reported (Gómez-Marín et al., 2010; Tarangini et al., 2013).

SEM image of melanin extracted from sunflower testae
Antioxidant activity
DPPH radical scavenging activity Antioxidant properties, especially radical scavenging activities, are important due to the deleterious role of free radicals in foods and biological systems. Excessive formation of free radicals accelerated lipids oxidation in foods and induced severe damage on adjacent biological macromolecules (Peksel et al., 2010). DPPH assay is the simplest and the most accurate method to evaluate the radical scavenging ability of antioxidants in all methods. In the solution, antioxidants interacted with DPPH radicals and transferred an electron or hydrogen atom to the radical, thus neutralizing the free radical character (Dasgupta et al., 2004). From Fig. 5a, the melanin extracted from sunflower seek husk has a significant ability to remove DPPH. Moreover, the ability to scavenge DPPH increased with an concentration increase and exhibited a positive correlation relationship. However, ascorbic acid used as positive control, was relatively more stronger than that of melanin and the synthetic melanin was similar with that of melanin. When the concentration was 2 mg/ mL, DPPH scavenging rates of the melanin reached 88.95% and slightly lower than that of ascorbic acid (91.71%), and there were significant difference between melanin and ascorbic acid in the concentrations tested (p < 0.05).

Biological activities of melanin extracted from sunflower testae. (a) DPPH. scavenging rate; (b) O2-. scavenging rate; (c) Hydroxyl scavenging rate; (d) Reducing power; (e)Removal rate of Pb2+, Cr3+ and Cu2+; (f)Survival rates of Bacillus thuringiensis.
Superoxide radical (O2−.) scavenging activity Although the superoxide was a relatively weak oxidant, and it could also indirectly induce lipid peroxidation as a result of hydrogen peroxide formation, creating precursors of the hydroxyl radical (Biswas et al., 2013). However, hydroxyl radicals was the most strong in all radicals. The scavenging ability of the superoxide radical for melanin and ascorbic acid are shown in Fig. 5b. The melanin extracted from sunflower seeds husk was found to be a moderate scavenger of superoxide radical generated in vitro, and was higher than that of the other reported (Manivasagan et al., 2013). However, ascorbic acid and synthetic melanin possessed higher (p < 0.05) superoxide radical scavenging activities than the melanin tested in a concentration dependent manner.
Hydroxyl radical scavenging activity Hydroxyl radical was the most active and the most harmful free radicals in all the reactive oxygen species known and could react with liposomes, peptides, proteins, DNA, especially thiamine and guanosine in the organism (Jin et al., 1996). The melanin showed the hydroxyl radical scavenging activity at different concentrations 0.1–2 mg/mL in a dose dependent manner (Fig. 5c). The hydroxyl radical scavenging activities of melanin increased with the increase of the concentration, and exhibited a good dose-effect relationship (y = 12.582x − 2.615, R2 = 0.9873). When the concentration was 0.l mg/mL, the hydroxyl radical scavenging rate of the melanin was only 9.42% and weakest in the defined range of the melanin dose. And then those of the melanin were enhanced in turn with the increase of melanin dose in the defined range. At last, that of the melanin at the concentration of 2 mg/mL reached 58.94% and was strongest in the range of melanin dose detected. However, all the hydroxyl radical scavenging activities of the melanin or synthetic melanin were significantly lower than those of ascorbic acid at the concentration of 0.1–2 mg/mL (p < 0.05).
Reducing power The reducing property was generally linked with reductant. The reductants, which showed the antioxidant activity, could donate the hydrogen atom to the free radical and break the chain reaction of the free radical. Moreover, the reductants also reacted with certain precursors of peroxide, thus preventing peroxide formation (Manivasagan et al., 2013). Therefore, the reducing power was an important index of antioxidant properties. Fig. 5d showed the reductive capabilities of the melanin compared to ascorbic acid and the synthetic melanin. The data of the reducing power in the melanin suggested that it was likely to contribute significantly towards the observed antioxidant effect. Like the antioxidant activity, the reducing power of the melanin increased with increasing amount of the melanin. However, the reducing power of ascorbic acid was relatively more pronounced than that of the melanin and the synthetic melanin.
Metal ion absorption activity The removal rates of metal ions by the melanin were similar among metal ions tested, increased with the amount of melanin and were higher than those of metal ions by the synthetic melanin (Fig. 5e). However, the removal rates of Cu2+ and Pb2+ were higher than that of Cr3+ in the same condition. The removal rate of Pb2+, Cu2+ and Cr3+ were improved from 39.29%, 19.48%, 6.82% to 73.14%, 84.36%, 47.85%, respectively, with the amount of melanin raised from 5 mg to 100 mg, which was due the increase of absorption site and absorption surface (Mall et al., 2006), and then the removal rate was improved with the addition of melanin. Nevertheless, the removal rate of melanin per unit mass decreased with the increase of melanin (data not shown).
Anti-radiation activity The survival rates of Bt mixed with the melanin or the synthetic melanin were significantly improved and the survival rates of Bt mixed with the melanin were higher than those of Bt mixed with the synthetic melanin (Fig. 5f). For example, when the irradiation duration was 5 min,10 min and 20 min, log A values of the Bt were increased by 22.83%, 54.77% and 134%, respectively, compared with those without melanin, indicating that melanin had significant anti-radiation effect on Bt, which were similar to the anti-radiation effect of melanins reported previously (Ye et al., 2014).
The importance of pigment additives for the safety of food, cosmetics and others has been increasingly recognized and it was emphasized that melanin deserved a better outline than that of the pathology and ecology in medicine. Melanin was considered as an effective protective agent for light, cosmic, nuclear, UV and ionizing radiation, X-ray, r-ray, drought, temperature extremes in the environment. Due to the high diversity of chemical structure of melanin and biological sources for melanin production, there is no common standard method for melanin isolation and purification. However, the extraction and purification of melanin mainly depended on one of the solubility characteristics, which was soluble in the alkaline solution and insoluble in the acidic solution. In the study, the method was developed for the melanin isolation and purification from sunflower testae. The acid hydrolysis was used to remove carbohydrates and proteins combing to melanin. Organic solvents were employed for washing away lipids. Multiple precipitations and sephadex G-50 were employed to improve the homogeneity.
Melanin extracted from sunflower testae showed all the physical and chemical properties which was common to synthetic melanin and other natural melanin (Wang et al., 2006; Sava et al., 2001a, 2001b; Tu et al., 2009). The property of the melanin was further confirmed by the spectral characteristics. The UV-vis spectrum was typical of the absorption plot of melanin (Suryanarayanan et al.,). The absorbance was strong in the UV region but progressively decrease as the increase of wavelength, which attributed to the complex conjugated structures of melanin (Cockell et al., 1999). The decrease in the absorption with increasing wavelength is almost lenear. Though the slope did not distinguish the types of different melanin, the gradient −0.0030 for the melanin of sunflower testae was similar to those of melanins extracted from the aleuriospores of Epicoccum nigrum Link (−0.0015), sclerotia of Colletotrichum coccodes S. Hughes (−0.0026), Ophiocordyceps sinensis (−0.0019) and synthetic melanin (−0.0017) (Dong et al., 2012). IR spectrum also displayed all the basic characteristics of melanin and some minor differences were due to the diversity of melanin (Kumar et al., 2011; Madhusudhan et al., 2015). The peak of the EPR spectrum was ascribed to the stable free radical population trapped in the melanin, which was the defining feature of melanin (Kumar et al., 2011; Enochs et al., 1993; Aghajanyan et al., 2005). By elemental composition analysis C, H, N, O and S atoms were detected, and then the melanin extracted from sunflower testae was concluded as one of allomelanins or phaeomelanins, and not eumelanins due to the present of S and chemical diversity of melanin (Sun et al., 2016b; Ye et al., 2014; Tarangini et al., 2013). The NMR spectroscopy of melanin could not be performed due to the scarce solubility, presence of free radicals and molecular complexity which caused the limitation in deriving the structural information. The determination of the structure was also a target of our project in the future.
Melanin had a wide spectrum of biological activity based on the antioxidant properties which were important in health care, functional food production, and so on. Antioxidants provided effective protection to DNA, protein and many other biologically important compounds from damage induced by UV radiation (Duan et al., 2007; Okunieff et al., 2008). The antioxidant activity depended on the redox state caused by the phenol - quinone structure in melanin (Cuevas-Juárez et al., 2014). The effect of melanin on the survival of Bt under UV radiation was mainly due to the extracellular redox buffer which could neutralize oxidants generated by environmental stress. The relation between melanin and mechanism was underway in this laboratory. Therefore, melanin from sunflower was considered as an efficient scavenger for reactive oxide species, a powerful chelator for metal ions, and an effective UV protective agent. The high absorption ability of melanin to the metal ions was due to the functional groups providing an array of multiple nonequivalent binding sites (Fogarty et al., 1996), which could confer a survival advantage. If metal ions were toxic to the organism, the melanin could prevent their entry to cell. If metal ions were essential to cell physiology and were present in low amounts in the environment, then melanin might concentrate them which made them more available to cell (Butler et al., 1998).
In conclusion, the pigment extracted from sunflower testae was confirmed as melanin based on the UV, FT-IR, EPR spectra and physicochemical analysis. Moreover, the melanin showed the important biological activities in anti-oxidation, metal ions removal and anti-radiation. Therefore, the melanin could be a promising natural antioxidant or anti-radiation. The biological roles indicated that the melanin could potentially be used as antioxidant, UV protective agent and biosorbent for Pb2+, Cu2+ and Cr3+ removal from aqueous solutions in the food, cosmetic and metal ions removal or extraction industries. Meanwhile, the melanin production would protect sunflower testae from incineration and landfill resulting in environment pollution.
Acknowledgment This work was financially supported by Jiangsu Province Natural Science Research Foundation (BK20150422), Yancheng City Agricultural Science and Technology Innovation Fund Project (YKN2014020), Jiangsu Provincial Key Laboratory of Coastal Wetland Bioresources and Environmental Protection Fund Project (JLCBE13004), Jiangsu Key Laboratory for Bioresources of Saline Soils Fund Project (JKLBS2014027). We thank Xiaoyan Jiang and Kaifeng Yan for assistance with various experiments. We also thank Prof. Zhenghao Fei and Prof. Zhenxing Li for allowing us to use their instruments.