2018 年 24 巻 6 号 p. 1039-1047
2018 年 24 巻 6 号 p. 1039-1047
Dried jujube fruit has been wildly used as flavoring in food preparation and food industry because of its unique and exquisite flavor. However, there is limited information available on the formation and alteration of jujube flavor during drying and storage. In this study, the effects of drying and storage on the volatiles in jujube fruit were investigated by electronic nose and GC-MS. Electronic nose and GC-MS could distinguish different jujube samples clearly; the ester and aldehydes showed significant decreases during drying, while furans and pyrazines were generated and increased with heat time; the esters, aldehydes, furans and pyrazines showed significant decreases, and some of them even disappeared during storage, while acids and terpenes increased with the extension of storage. Based on the present results, furans and pyrazines, generated during drying, are the predominant components of the unique and exquisite flavor of dried jujube, and these compounds could be negatively influenced because of evaporation, oxidation and degradation of jujube volatiles during storage.
Jujube (Zizyphus jujuba Mill) belongs to Rhamnaceae family, and it is one of the most important species for fruit production. It is wildly distributed throughout the warmer parts of Asia, northern Africa, the Middle East, southern Europe, and south-western USA (Almansa, et al., 2016). Jujube is believed to have originated from China, which owns a history of more than 4000 years and over 700 cultivars (Wojdylo, 2016). Its annual yield reached 7,345,300 tons, with an approximate total area of 2.8 million hectares cultivation in China (Ji, et al., 2017). Jujube fruit doesn't only contain high amount of nutrients components, such as in sugars, protein, organic acids, vitamins and minerals (Li, et al., 2007), but it is also a good source of phytochemicals such as vitamins, cyclic adenosine monophosphate, alkaloids, saponins, polyphenols (Gao, 2012), and polysaccharides (Liu, et al., 2015). It has been commonly used as food and food flavoring as well as traditional medicine in Asian countries for thousands years (Zang, et al., 2015). However, like most other fruits, jujube fruit has high moisture amount and is highly perishable, associated with a short harvest season. Because drying is commonly used in the fruit processing industry to extend their shelf life, the dried jujube fruit, to date, has been the predominant commercial commodity available throughout the year (Kou, 2015).
Except for the nutritional and functional properties, the unique flavor of jujube fruit is another important factor attracted consumer's preference. Dried jujube fruit and its extract have been wildly used as flavoring in food preparation and food industry. In fact, jujube flavor commonly means the flavor of dried jujube fruit or its extract, rather than that of fresh one. Additionally, it is well known that the flavor preference of food commonly decreases with the extension of the storage period (Mayuoni-Kirshinbaum et al., 2013). Therefore, it is necessary to pay attention to the formation jujube flavor during drying process and its alteration during storage.
Compared to the non-volatile compounds, the volatile compounds of jujube fruit have attracted less researcher's attention, and only few papers have reported the volatiles of jujube fruit. Hernández and Galindo (Galindo, et al., 2015; Hernandez, et al., 2016) reported that there were less than twenty volatile compounds in fresh jujube fruit from four Spain jujube varieties, and the most abundant compounds were aldehydes, such as hexanal, nonanal and benzaldehyde, while Wang (Wang, et al., 2016) reported that forty-six volatiles were identified from fresh jujube fruit, and the major volatile components were acids, such as acetic acid, dodecanoic acid and n-decanoic acid. Reidel (Bandeira, et al., 2017) reported there were 144 volatiles in jujube samples, and the fragrance bouquet of ripe fruits mainly constituted of monoterpenes (79.6%). These researches mainly focused on the volatiles of fresh fruit, while neglected those of dried fruit. Although Wang (Wang, et al., 2016) also reported that acetic acid, dodecanoic acid, furfural, and 5-hydroxymethylfurfural were the predominant volatiles of dried jujube fruit, it is difficult to make sense that acetic acid are characteristic aroma components of dried fruit, because acetic acid generates pungent and sour flavor rather than sweet and pleasant toasting flavor. However, to date, it is still obscure about the characteristic aroma components and their formation in dried jujube fruit, which is of vital importance for understanding the characteristic flavor formation of dried jujube fruit and developing methods for jujube processing and storage.
In recent years, the use of gas chromatography-mass spectrometry (GC-MS) and electronic nose have been the main methods used to analyze food flavor (Chen, et al., 2016; Wu, et al., 2017). GC-MS has mainly focused on the measurement of certain volatile components, while electronic nose provides a pattern output showing the component combinations contributing to a defined smell (Cheng, et al., 2015). Therefore, the volatile compounds of fruits and vegetables were commonly analyzed and characterized using electronic nose and GC-MS together.
The objectives of this study were to investigate the feasibility of using an electronic nose to differentiate jujube fruit during drying and storage by discriminant factor analysis (DFA) and to determine their aroma profiles of by GC-MS combined with principal component analysis (PCA). These results will be helpful to identify the characteristic aroma components, and understand the evolution of volatile components in jujube fruit during drying and storage.
Chemicals A standard solution of n-alkanes (C8–C20, 40 mg.L−1 each component in hexane), purchased from Sigma–Aldrich (St. Louis, MO), was used for retention index calculation. The cyclohexanone, used as the internal standard, was obtained from J&K Chemical Ltd (Shanghai, China).
Samples Jujube fruit (Zizyphus Jujuba cv. Junzao) was harvested at commercial maturity state from a local orchard in Xinjiang, China. The samples were picked with intact appearance and similar morphological properties. All jujube fruits were washed with tap water and drained well. Then, these fruits were randomly divided into five sections (about 3 kg per section): one was regarded as fresh sample (FF); one, dried by hot air at 55°C for 12 h, was regarded as semi-dried fruit (DF1); one, dried by hot air at 55°C for 24 h, was regarded as dried fruit (DF2); one, dried by hot air at 55°C for 24h and stored at ambient temperature for 6 months, was regarded as stored fruit (SF1); one, dried by hot air at 55°C for 24h and stored at ambient temperature for 12 months, was regarded as stored fruit (SF2). All samples were halved for removing kernels, then freeze-dried (FreeZone 6, Labconco, USA) and homogenized with a domestic blender (JYL-C022E, Jiuyang, China), and packed in polyethylene bags and stored at −20°Cfor analysis.
Electronic nose An electronic nose (Fox 4000, Alpha M.O.S., Toulouse, France) equipped with 18 metal oxide sensors was used to discriminate the odors produced by jujube fruit during drying and storage. The electronic nose consists of a sampling apparatus, a detector unit containing an array of sensors, air generator equipment and computer-controlled pattern recognition software. The sensor array used was composed of 18 metal oxide sensors enclosed in three chambers, which are LY2/LG, LY2/G, LY2/AA, LY2/GH, LY2/gCTl, LY2/gCT, T30/1, P10/1, P10/2, P40/1, T70/2, PA/2, P30/1, P40/2, P30/2, T40/2, T40/1 and TA/2.
Each sample (1.0 g) was weighed in a 20 mL headspace vial and equilibrated at 45°C for 30 min for headspace volatiles generation. Then, 2000 µL of headspace air was manually injected into electronic nose by a syringe. The measurement time was 120 s. The interval for data record was 1s. The cleaning time was set to 240s. All measurement procedures were performed at 25 ±1°C. All samples were repeated more than four times, and the data was analyzed by the E-nose software after the measurement was completed.
Headspace solid-phase microextraction (HS-SPME) The SPME fiber (50/30 µm DVB/CAR/PDMS) was purchased from Supelco (Bellefonte,PA), and it was previously activated before sampling according to the manufacturer's instructions. The jujube sample (1.5 g) was added to a 15 mL vial tightly sealed with a polypropylene cap and a polytetrafluoroethylene (PTFE)/silicon septum, and left for 20 min at 50°C to allow for the equilibration of the volatiles in the headspace. After the equilibration, the septum covering each vial was pierced with a SPME needle and the fiber was exposed to the headspace for 30 min.
GC-MS The volatiles adsorbed by the fiber were thermally desorbed in the hot injection port of a gas chromatograph 7890 coupled to a quadrupole mass spectrometer Series MSD 5975 (Agilent Technologies, Palo Alto, CA, USA) for 3 min at 250°C (splitless mode). VF-WAXms capillary column (60 m×0.25 mm i.d., 0.25 µm) (Agilent Technologies, EU) was used. The carrier gas was high purity helium, at a flow rate of 1.0 mL/min. The initial oven temperature was 40°C, which was ramped up at 10°C /min to 80°C. Then it is programmed to rise a temperature of 200°C at 3°C /min. Finally, it was ramped up at 5°C /min to 240°C and held there for 10 min. The GC-MS interface was heated at 280°C with the actual temperature reaching 180°C in MS source and 150°C in MS-quadrupole. The electron impact energy was set at 70 eV, and data were collected in the range of 30–500 atomic mass units (amu). Compounds identification was based on mass spectra by comparison with the NIST11library and Kovats indices comparison with online database of Volatile Compounds in Food 16.3. Each sample was analyzed in duplicate.
Statistical analysis The results were expressed as mean ± SD (standard deviation). All data was subjected to one-way analysis of variance using the SPSS statistical software (Version: 20.0, USA), and PCA of volatile compounds was performed using Unscrambler 10.1 (CAMO AS, Trondheim, Norway) software.
Electronic nose analysis The volatile profile of jujube fruit subjected to the treatment of drying and storage was performed by Fox 4000 electronic nose. The response data generated by 18 sensors of electronic nose was collected and transformed into radar graph (Fig. 1). The results showed that the outline of jujube aroma altered during drying and storage, the variation of jujube flavor had significant influence on response values of the sensors TA/2, T40/2, P30/2, P40/2, P30/1, PA/2, T70/2, P40/1, P10/2, P10/1 and T30/1. The response values of these sensors decreased with the extending drying time, while the response values of these sensors increased with the prolonging of storage time.
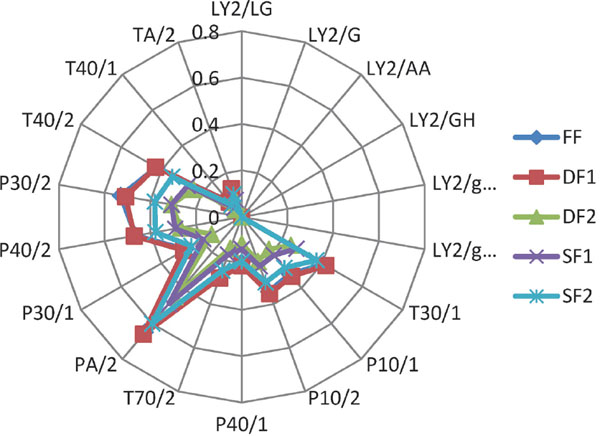
Radar graph of jujube samples detected by Electronic nose
For improving the interpretation of the results from electronic nose, DFA was applied to identify patterns of correlation with individual composition variables involved in the discrimination among jujube samples during drying and storage (Fig. 2). The variance contribution rates of the first and second DFs were 51.09% and 43.51%, respectively. The accumulative contribution rate of the first two DFs was 94.60% (more than 85%), indicating that the two discriminant factors include the most information of the response values of electronic nose. As it can be seen from Fig. 2, electronic nose can clearly distinguish FF, DF1, DF2, SF1 and SF2, which located in different areas on the plane. These results suggested that the flavor volatiles of jujube fruit were obviously influenced by drying process and storage, and electronic nose has the potential to be applied in quality monitoring of jujube fruit during drying and storage.
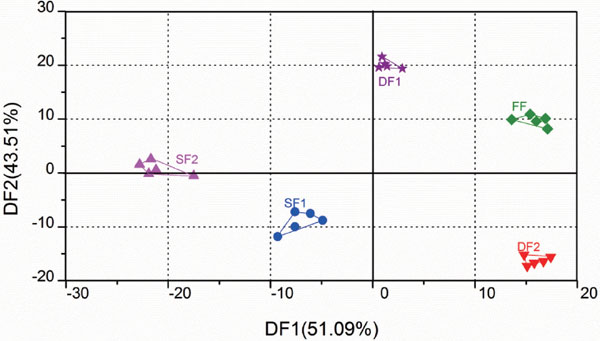
DFA of E-nose analysis of jujube fruit samples
GC-MS analysis The code, name, retention time, Kovats index and content of the detected compounds from jujube samples are shown in Tab.1 and Tab.2. As shown in Tab.1, there are totally 58 identified volatile compounds in jujube samples, including 2 alcohols, 2 aldehydes, 5 ketones, 10 acids, 21esters, 8 furans, 2 pyrazines, 4 terpenes and 4 others. The effect of drying and storage had significant influence on the aroma composition and the volatile levels of jujube fruit. In the present study, the volatiles of fresh jujube fruit (FF) consisted of thirty-seven volatiles, and its predominant volatiles were benzaldehyde, hexanoic acid, ethyl heptanoate and ethyl benzoate. These results are a little different from those reported by previous literature (Bandeira, et al., 2017; Galindo, et al., 2015; Hernandez, et al., 2016; Wang, et al., 2016), but they are in agreement with the fact that a full mature jujube fruit tastes a little bit like an apple (Liu, 2016), and ethyl heptanoate and ethyl benzoate were identified as the predominant volatiles of apple (Both, et al., 2014; Yauk, et al., 2017).
| Code | Name | Retention time | Kovats indices | Concentration (µg/g) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Exp | Ref | FF | DF1 | DF2 | SF1 | SF2 | |||
| Alcohols | |||||||||
| A1 | 1-Octen-3-ol | 24.826 | 1449 | 1446 | 0.20±0.08b | 0.34±0.05b | 0.64±0.19a | NDc | NDc |
| A2 | Benzyl alcohol | 40.887 | 1892 | 1868 | 0.26±0.05a | 0.33±0.02a | 0.42±0.12a | 0.31±0.06a | 0.23±0.10a |
| Aldehydes | |||||||||
| B1 | Nonanal | 23.247 | 1407 | 1391 | 0.23±0.17a | 0.39±0.17a | 0.45±0.15a | 0.35±0.13a | 0.42±0.18a |
| B2 | Benzaldehyde | 29.094 | 1563 | 1520 | 11.83±1.86a | 8.87±1.65b | 6.32±0.78c | 3.20±1.13d | 2.59±1.08d |
| Ketones | |||||||||
| C1 | 6-Methyl-5-hepten-2-one | 21.253 | 1352 | 1339 | 0.31±0.02a | NDb | NDb | NDb | NDb |
| C2 | 6-Methyl-3,5-heptadiene-2-one | 30.809 | 1609 | 1599 | NDb | 0.12±0.08a | 0.09±0.13a | 0.06±0.09a | NDb |
| C3 | Acetophenone | 33.266 | 1675 | 1635 | 0.25±0.04a | 0.16±0.05a | 0.07±0.09a | 0.05±0.07a | NDb |
| C4 | (E)-β-Ionone | 43.077 | 1958 | 1931 | 0.03±0.04a | NDb | NDb | NDb | NDb |
| C5 | 2-Acetylpyrrole | 44.157 | 1992 | 1982 | NDc | NDc | NDc | 0.24±0.02b | 0.43±0.16a |
| Acids | |||||||||
| D1 | Acetic acid | 25.491 | 1467 | 1446 | 0.37±0.29b | 0.53±0.14b | 0.63±0.18b | 1.82±0.45a | 2.29±0.53a |
| D2 | Pentanoic acid | 36.056 | 1753 | 1732 | 0.08±0.12b | 0.26±0.14a | 0.28±0.26a | 0.39±0.02a | 0.29±0.12a |
| D3 | (E)-2-Butenoic acid | 37.605 | 1796 | NDb | 0.04±0.06a | 0.07±0.02a | 0.08±0.01a | 0.06±0.03a | |
| D4 | Hexanoic acid | 39.741 | 1859 | 1841 | 4.71±0.31a | 3.78±0.24b | 3.04±0.37c | 3.36±0.64bc | 3.75±0.21b |
| D5 | Heptanoic acid | 43.279 | 1965 | 1936 | 0.22±0.12c | 0.78±0.12b | 0.74±0.13b | 1.90±0.35a | 2.02±0.17a |
| D6 | Octanoic acid | 46.567 | >2000 | 2052 | 0.34±0.09d | 0.81±0.35c | 0.77±0.21c | 1.33±0.01b | 2.09±0.39a |
| D7 | 2-Heptenoic acid | 47.303 | >2000 | NDb | 0.15±0.17a | 0.23±0.08a | 0.21±0.03a | 0.22±0.16a | |
| D8 | Nonanoic acid | 49.434 | >2000 | 2158 | 0.14±0.12b | 0.27±0.19b | 0.28±0.09b | 0.22±0.11b | 0.44±0.20a |
| D9 | n-Decanoic acid | 51.962 | >2000 | 2272 | 2.70±0.43a | 1.79±0.23c | 1.15±0.13d | 0.96±0.06d | 1.63±0.16b |
| D10 | Benzoic acid | 57.001 | >2000 | 2419 | 1.69±0.09d | 2.19±0.17c | 2.02±0.29c | 4.05±0.18b | 5.16±0.36a |
| Esters | |||||||||
| E1 | Ethyl acetate | 10.095 | <800 | 883 | 2.83±0.32a | 2.19±0.26b | 1.64±0.16c | 1.22±0.33c | 0.50±0.71d |
| E2 | Ethyl butyrate | 12.362 | 1047 | 1034 | 1.32±0.45c | 1.17±0.65c | 2.89±0.37a | 2.13±0.45b | 0.85±0.20d |
| E3 | Ethyl pentanoate | 14.677 | 1146 | 1131 | 4.39±0.19b | 4.89±0.18a | 5.06±0.78a | 5.78±0.48a | 3.18±0.10c |
| E4 | Ethyl hexanoate | 17.543 | 1243 | 1232 | 29.69±2.68a | 22.79±2.33b | 20.97±1.66b | 17.93±1.36c | 11.12±0.19d |
| E5 | Ethyl heptanoate | 20.861 | 1341 | 1329 | 2.44±0.09a | 2.53±0.31a | 2.34±0.18a | 2.24±0.16a | 2.04±0.29b |
| E6 | Ethyl 2-hexanoate | 21.425 | 1357 | 1336 | 0.19±0.04c | 0.17±0.25c | 0.29±0.03b | 0.34±0.02ab | 0.42±0.08a |
| E7 | Ethyl octanoate | 24.517 | 1441 | 1433 | 1.36±0.08bc | 1.58±0.12b | 1.86±0.11a | 1.30±0.10bc | 1.07±0.53c |
| E8 | Ethyl nonylate | 28.286 | 1541 | 1539 | 0.27±0.08c | 0.24±0.02c | 0.35±0.06b | 0.25±0.01c | 0.50±0.12a |
| E9 | Ethyl 4-oxo-pentanoate | 31.278 | 1621 | 1600 | NDb | NDb | NDb | 0.25±0.07a | 0.31±0.11a |
| E10 | Ethyl decanoate | 32.073 | 1643 | 1638 | 1.89±0.31c | 2.35±0.45b | 3.02±0.55a | 2.15±0.06b | 1.60±0.41c |
| E11 | Ethyl benzoate | 33.711 | 1687 | 1661 | 14.00±1.54a | 10.89±0.44b | 6.71±0.64c | 3.06±0.53d | 1.77±0.31e |
| E12 | Ethyl undecanoate | 35.741 | 1744 | 1734 | 0.24 ±0.08b | 0.23±0.05b | 0.34±0.23a | 0.04±0.06d | 0.16±0.06c |
| E13 | Ethyl benzeneacetate | 37.747 | 1800 | 1785 | 0.08±0.05a | 0.05±0.07a | 0.12±0.03a | 0.04±0.06a | 0.04±0.06a |
| E14 | Methyl dodecanoate | 37.949 | 1806 | 1798 | 0.18±0.05b | 0.26±0.09b | 0.39±0.05a | 0.25±0.04b | 0.31±0.10a |
| E15 | Ethyl dodecanoate | 39.314 | 1846 | 1842 | 2.60±0.52a | 1.54±0.11b | 1.71±0.80b | 0.61±0.11c | 0.26±0.23d |
| E16 | Ethyl benzenepropanoate | 41.207 | 1901 | 1887 | 2.18±0.79a | 2.22±0.68a | 1.89±0.72ab | 0.74±0.15v | 1.60±0.65b |
| E17 | Ethyl tetradecanoate | 49.956 | >2000 | 2040 | 0.55±0.15a | 0.26±0.15b | 0.27±0.11b | NDc | NDc |
| E18 | Ethyl 9-tetradecenoate | 47.107 | >2000 | 2.27±0.79a | 0.73±0.06b | 0.30±0.14c | 0.10±0.14d | NDe | |
| E19 | Methyl hexadecanoate | 50.49 | >2000 | 2207 | 0.07±0.10a | 0.11±0.15a | NDb | NDb | NDb |
| E20 | Ethyl hexadecanoate | 51.333 | >2000 | 2248 | 0.79±0.09a | NDd | 0.03±0.05c | NDd | 0.12±0.05b |
| E21 | Ethyl palmitoleate | 52.295 | >2000 | 2275 | 1.47±0.40a | 0.13±0.18b | 0.10±0.14b | NDc | 0.12±0.13b |
| Furans | |||||||||
| F1 | Furfural | 26.055 | 1482 | 1461 | NDd | 0.22±0.17c | 0.69±0.42b | 0.93±0.17a | 0.25±0.22c |
| F2 | 1-(2-furanyl)-Ethanone | 27.657 | 1524 | 1496 | NDc | 0.05±0.04b | 0.15±0.06a | 0.12±0.08a | NDc |
| F3 | 5-Methylfurfural | 30.233 | 1593 | 1568 | NDc | 0.06±0.09b | 0.17±0.01a | 0.25±0.01a | 0.17±0.06a |
| F4 | 5-Ethyldihydro-2(3H)-furanone | 35.296 | 1731 | 1691 | NDc | 0.11±0.7b | 0.26±0.05a | 0.19±0.08a | 0.04±0.06b |
| F5 | Dihydro-5-propyl-2(3H)-furanone | 38.81 | 1831 | 1812 | NDb | 0.14±0.4a | 0.20±0.02a | 0.15±0.02a | 0.11±0.05a |
| F6 | Dihydro-5-pentyl-2(3H)-furanone | 42.59 | 1922 | 2025 | NDb | 0.09±0.06a | 0.13±0.03a | 0.09±0.05a | 0.07±0.03a |
| F7 | 5-Heptyldihydro-2(3H)-furanone | 46.128 | >2000 | 2265 | NDc | 0.06±0.08b | 0.10±0.02ab | 0.14±0.06a | 0.16±0.07a |
| F8 | 5,6,7,7a-Tetrahydro-4,4,7a-trimethyl-2(4H)-benzofuranone | 54.698 | >2000 | 2272 | NDc | NDc | NDc | 0.09±0.13b | 0.17±0.02a |
| Pyrazines | |||||||||
| G1 | 2-Ethyl-6-methyl-pyrazine | 23.034 | 1401 | 1384 | NDc | 0.16±0.03b | 0.45±0.03a | NDc | NDc |
| G2 | Trimethyl-pyrazine | 23.692 | 1419 | 1398 | NDc | 0.03±0.01b | 0.08±0.01a | NDc | NDc |
| Terpenes | |||||||||
| H1 | β-Myrcene | 15.442 | 1174 | 1161 | ND | ND | ND | 2.42±0.42 | 0.53±0.75 |
| H2 | d-Limonene | 16.849 | 1222 | 1205 | 1.79±0.04c | 1.30±0.22c | 0.94±0.31c | 7.80±1.75a | 5.98±0.92b |
| H3 | γ-Terpinene | 18.25 | 1265 | 1245 | NDc | NDc | NDc | 1.41±0.34a | 0.11±0.16b |
| H4 | Styrene | 18.724 | 1280 | 1257 | 0.17±0.02a | 0.15±0.05a | 0.14±0.4a | 0.11±0.04a | 0.15±0.03a |
| Others | |||||||||
| I1 | Naphthalene | 36.762 | 1773 | 1746 | NDb | 0.06±0.02a | 0.08±0.02a | 0.05±0.01a | 0.09±0.03a |
| I2 | (+)-δ-Cadinene | 36.928 | 1777 | 0.80±0.28a | 0.24±0.08b | 0.07±0.05c | 0.04±0.02c | 0.06±0.01c | |
| I3 | 2-Methoxy-phenol | 40.436 | 1879 | 1861 | NDb | NDb | NDb | 0.13±0.19a | 0.31±0.08a |
| I4 | 2,6-Bis(1,1-dimethylethyl)-4-(1-oxopropyl)phenol | 41.949 | 1924 | NDb | NDb | NDb | 0.09±0.13a | 0.03±0.04a | |
1) Exp was caculated using C8-C20 as external standards
2) Ref was obtained from the online database of Volatile Compounds in Food 16.3 (http://www.vcf-online.nl/VcfCompoundSearch.cfm)
3) ND: Not detected
4) *Mean ± SD. Values in each row with different letters are significantly different (P < 0.05)
| Item | Content (µg/g) | ||||
|---|---|---|---|---|---|
| FF | DF1 | DF2 | SF1 | SF2 | |
| Alcohols | 0.46±0.13c | 0.67±0.07b | 1.06±0.31a | 0.31±0.06cd | 0.23±0.10d |
| Aldehydes | 12.06±2.03a | 9.16±1.83b | 6.77±0.93c | 3.55±1.26d | 3.01±1.26d |
| Ketones | 0.50±0.10a | 0.28±0.13ab | 0.16±0.22b | 0.35±0.18a | 0.43±0.16a |
| Acids | 10.25±1.55c | 10.60±1.61c | 9.21±1.76c | 14.32±1.86b | 17.95±2.33a |
| Esters | 68.81±9.17a | 54.33±6.56b | 50.28±6.81b | 38.43±3.98c | 25.97±4.33d |
| Furans | 0 | 0.73±0.45b | 1.70±0.61a | 1.93±0.60a | 0.97±0.49b |
| Pyrazines | 0 | 0.19±0.04b | 0.53±0.04a | 0 | 0 |
| Terpenes | 1.96±0.06c | 1.45±0.27c | 1.08±0.71c | 11.74±2.75a | 6.77±1.88b |
| Others | 0.80±0.28a | 0.30±0.10b | 0.15±0.07b | 0.31±0.35b | 0.49±0.16b |
| Total | 94.93±13.32a | 77.81±11.06b | 70.94±11.46b | 70.97±11.04b | 55.82±10.17c |
1) Values in each row with different letters are significantly different (P < 0.05)
After the drying process, the concentration of most volatile components changed significantly when compared with the fresh jujube fruit. Quantitatively, the total amount of volatiles decreased by about 20% in dried jujube fruit. In fact, aldehydes and esters decreased with drying time, while furans and pyrazines generated and increased with drying time. Volatiles losses were commonly occurred in fruit drying process, because heated air not only vaporizes water but also volatile compounds (Bonneau, et al., 2016; Sunthonvit, et al., 2007). Heat treatment markers, such as furfural, 5-methylfurfural and pyrazines, were found in dried jujube fruit. Generation of 5-methylfurfural and furfural in raisins, dried plums, dried mango and dried nectarines has been also reported in published literature (Bonneau, et al., 2016; Wang, et al., 2017). Oxygen-containing heterocyclic compounds, including furans and furan derivatives, are well-known products of Maillard reactions. The formation of pyrazines has been known to be formed by the condensation reaction of α-amino ketones, produced by the Strecker degradation of amino acids, with reducing sugars (Cho, et al., 2010). Also, these compounds can be formed when sugar or sugar degradation products are heated with amino acids (Ara, et al., 2017). Furans and pyrazines didn't occur in fresh jujube fruit, while only generated in the drying process, thus furans and pyrazines may be recognized as the main contributors of aroma notes in dried jujube fruit.
On comparison with DF2, the total amount of volatiles showed no significant variation after six months of storage, while decreased by 19% after twelve months of storage. With an exception of terpenes and furans, these compounds showed an increase after six months of storage, and then showed a decrease after twelve months of storage. Basically, alcohols, aldehydes, pyrazines and esters decreased with extension of the storage period, while acids increased significantly. Evaporation is commonly recognized as the main factor for volatiles decrease during storage. Bahmanzadegan (Giovanelli, et al., 2017) found that the amount of volatiles with a minor molecular weight diminished by extending the storage time specifically at room temperature. He (He, et al., 2018) also found that the concentrations of alcohols and aldehydes of lemon-flavored hard tea decreased dramatically during storage. The volatiles could be formed through Maillard reactions, oxidation, degradation and other complex reactions (Deng, et al., 2015). This could explain why the concentration of acids increased during storage in this study. For instance, it belongs to typical oxidation reactions that the concentration of benzaldehyde decreased during storage, while that of benzoic acid increased. However, it is difficult to make sense why the concentration of d-limonene increased sharply after six months of storage, and then decreased after twelve months of storage. After twelve months of storage, the pyrazines disappeared and furans showed a significant decrease in SF2. This was further evidenced that pyrazines and furans are the characteristic aroma components of dried jujube fruit.
Principal component analysis (PCA) In order to visualize the overall effects of drying and storage on the volatiles of jujube fruit and identify the main constituents influencing the variability, the total data set of volatiles was analyzed by the technique of principal component analysis, which is a well-known statistical technique to visualize the resemblance and difference by reducing the dimensionality of numerical datasets (Jo, et al., 2013). Generally, the PCs have more than 85% cumulated reliability of the original dataset, then these PCs can be used to replace the original one (Cevoli, et al., 2011). In this study, Ninety-nine percent of the variability in the datasets could be explained by two principal PCs, whereas the first two principal components accounted for 72% and 27% of the total variance, respectively. As can be seen from the correlation loading plot (Fig. 3), the samples and indices can be appropriately divided into four groups. On the right plane, the esters and aldehydes obviously gathered around FF, and formed the group one. This suggested that esters and aldehydes should be the characteristic volatiles of fresh jujube fruit, which is in agreement with the fact that esters and aldehydes are the fruity flavor contributors of fresh fruits. DF1 and DF2, represented as group two, scattered on the right plane nearby the central, and pyrazines and few other volatiles gathered around them. SF1 and SF2, presented group three and group four, appeared on the bottom and top of left pane, respectively. Volatiles of acids, terpenes and furans mainly plotted in this area. From the amount and distance between sample and indices of view, benzaldehyde, ethyl heptanoate and ethyl benzoiate are the characteristic aroma components of fresh jujube fruit, and furfural, 5-ethyldihydro-2(3H)-furanone, 5-methylfurfural and 2-ethyl-6-methyl-pyrazine are the note aroma components of dried jujube fruit, and d-limonene, γ-terpinene, benzoic acid, octanoic acid, and acetic acid are the predominant components of dried jujube fruit after storage.
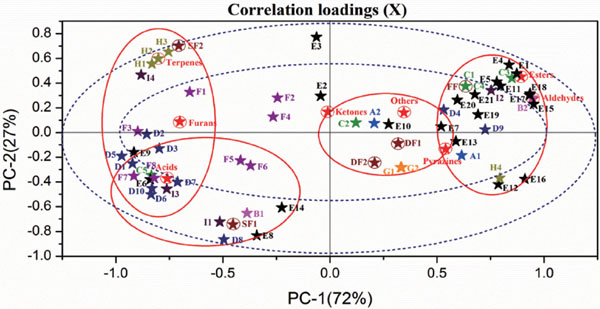
Correlation loading plots of principal component analysis (PCA).
In the present study, the effects of drying and storage on the volatile profiles of jujube samples were evaluated by GC–MS and electronic nose. From the present results of view, the techniques of electronic nose and GC-MS could distinguish fresh, dried and stored jujube fruit clearly. Accordingly, these techniques have potential to be applied in quality monitoring of jujube fruit during process and storage. Fresh jujube fruit mainly contained aldehydes and esters with benzaldehyde, ethyl heptanoate and ethyl benzoate being the predominant volatiles; Esters and aldehydes are also the major volatiles of dried jujube fruit, but its note aroma components should be pyrazines and furans such as furfural, 5-ethyldihydro-2(3H)-furanone, 5-methylfurfural and 2-ethyl-6-methyl-pyrazine; Terpenes, esters and acids are the major volatiles of dried jujube fruit experienced a period of time storage, and d-limonene, benzoic acid, acetic acid and octanoic acid could be regarded as indicators of storage duration. Summarily, fresh jujube fruit is characterized with fruity and sweet flavor, and its pleasant toasting flavor is formed during drying, but its note flavor will be negatively influenced because of evaporation, oxidation and degradation of jujube volatiles during storage. Therefore, to acquire good jujube note flavor, the future work should pay more attention to optimize the drying technology, choose suitable package material, and optimize storage conditions.
Acknowledgement This study was financially supported by the National Natural Science Foundation of China (No. 31460396) and the Projects of state's key R&D program of China (2016YFD0400301).