2018 年 24 巻 6 号 p. 1069-1081
2018 年 24 巻 6 号 p. 1069-1081
Daqu is an essential starter for the fermentation of Shanxi aged-vinegar. Despite numerous studies on the microbial diversity in Daqu, there is little information on the use of autochthonous yeasts originated from Daqu for vinegar fermentation. Herein, an ester-producing yeast strain, designated Y18 (33.48 g L−1 ester), was isolated from Daqu. The strain Y18 was identified as Candida ethanolica based on sequence analysis of the D1/D2 domain of the 26S rRNA gene, in combination with the morphological and physiological characteristics. The results of gas chromatography-mass spectrometry showed that inoculation of Y18 into the Daqu-based fermentation broth increased the relative abundances of total alcohols and total esters in the Jiulao compared to the non-inoculated control. In particular, the relative abundances of ethanol, isoamyl acetate, and ethyl acetate increased from 31.9% to 38.0%, 1.0% to 1.5%, and 9.8% to 14.5%, respectively. Five different aroma compounds (isoborneol ethanol, isobutanol, benzoic acid methyl ester, acetic acid 2-phenylethyl ester, and hexanoic acid phenyl ester) were detected in the Y18-fortified alcoholic fermentation broth compared to the non-inoculated control. After Y18 fortification, the levels of total acids and total esters in the vinegar increased from 37.42 and 15.30 g L−1 to 40.24 and 19.25 g L−1, respectively. The sensory analysis of Y18-fermented vinegar revealed no abnormal odors but distinct fruity and flowery aromas, with slightly sweet and lingering taste. In conclusion, the inoculation of C. ethanolica strain Y18 into the Daqu-based fermentation broth could markedly enhance the aroma of the Shanxi aged-vinegar by increasing the contents of total acids and total esters, and improve the vinegar quality by conferring the flowery and fruity flavors and slightly sweet, nut-like taste.
Vinegar is widely used in foods and commercially available around the world (Mazza et al., 2009). In Western countries, vinegar is commonly made from fruits, e.g., Italian balsamic vinegar and Spanish wine vinegar (Caligiani et al., 2007; González-Viñas et al., 1996). In China, vinegar is often produced from the natural fermentation of cereals, including sorghum, pea, barely, wheat bran, and rice hull (Nie et al., 2013). The quality of vinegar is directly related to the metabolites produced by the dominant fermenting microorganisms during the fermentation. The identification and characterization of the major fermenting strains and species is desirable to improve the stability of fermentation process and the flavor of vinegar (Hidalgo et al., 2010; Uysal et al., 2013; Trček et al., 2016).
A variety of local vinegars are produced in different regions of China (Wu et al., 2012). Shanxi aged-vinegar, with the history of more than 3,000 years (Shi, 1999), is a traditional product from Shanxi province, China. Due to its fine, distinctive flavor and multiple health benefits, Shanxi aged-vinegar is considered to be the best among the four most valuable Chinese vinegars. The spontaneous fermentation of Shanxi aged-vinegar involves complex traditional techniques listed in the “National Non-Material Cultural Heritage” item (Liu et al., 2004; Chen et al., 2013). The characteristics of this vinegar are dependent on the local water, soil, climate, and environment because all of these factors affect the microbial community composition of Daqu (Wu et al., 2004; Chen et al., 2009; Nie et al., 2013), a saccharifying, flavor-imparting, and fermenting agent in the traditional production of Shanxi aged-vinegar (Zheng et al., 2011).
As an industrial fermentation starter, Daqu is made from barely and pea by spontaneous growth of the natural occurring microorganisms, and contains more than 50 types of enzymes (Chen et al., 2009; Nie et al., 2013). The microorganisms in Daqu have evolved to be compatible with each other and formed a stable community (Wu et al., 2012). Several studies have evaluated the microbial community composition and dynamics during spontaneous fermentation induced by Daqu (Zheng et al., 2014; Li et al., 2013). As an important fermenting microorganism in Daqu, yeast is classified into the alcohol-producing and aroma-producing group. Saccharomyces cerevisiae, Hansenula anomala, and Candida berkhout are the main yeast species used for alcoholic fermentation (Mao et al., 1998; Chen et al., 1999; Wu, 2004). Members of the genera Hansenula, Torulopsis, and Candida species are the major yeasts contributing to aroma production (Zhong et al., 2015).
Saccharomyces cerevisiae is the most abundant alcohol-producing yeast species, accounting for 95% of the total yeast in alcoholic fermentation (Wu et al., 2012). Commercialized S. cerevisiae, an aroma-producing yeast, and Daqu are often added to increase the alcohol yield prior to the fermentation of acetic acid and to enhance the flavor of the Shanxi aged-vinegar. In addition, a mutant strain of Hansenula anomala, BY2, was reported to have a markedly increase on the alcohol yield (Li, 2011). Even though their prevalence is low, the aroma-producing yeast species, such as Kluyveromyces marxianus, Zygosaccharomyces rouxii, and Wickerhamomyces anomalus, were reported to considerably affect the quality and flavor of alcoholic beverages and vinegars (Zhong et al., 2015). However, the previous studies have mainly focused on the diversity of yeasts in Daqu (Zhong et al., 2015; Dong et al., 2016), and there is little information on the use of Daqu-originated autochthonous yeasts for the fermentation of the Shanxi aged-vinegar.
The aim of the present study was to isolate and screen an aroma-producing yeast strain from Daqu, re-introduce it for Daqu-based vinegar fermentation, and enhance the production of esters without negatively affecting the saccharifying and fermenting properties of Daqu. The introduction of the selected yeast strain for Daqu-based vinegar fermentation was expected to improve the quality of the vinegar notably in terms of aroma properties.
Yeast isolation from Daqu A semi-alcoholic fermented product usually named Jiulao in Chinese was used for yeast isolation. Generally, Jiulao is produced spontaneously after steamed sorghum is mixed with Daqu powder and water (Wu et al., 2012). Herein, Jiulao was used as the fermented starch material in the alcoholic fermentation. This process lasted for approximately 10 days, including 2 days of aerobic fermentation and 8 days of anaerobic fermentation. Five Jiulao samples were obtained from 10-cm below the leavening surface of the Daqu-based solid-state alcoholic fermentation broth at day 6 (Tongbao Vinegar Co., Ltd., Taigu, Shanxi, China).
Each sample (10 g) was mixed with 90 mL of sterile distilled water in a 150-mL Erlenmeyer flask by vortexing. Serially diluted suspensions (200 µL each) were plated on the Rose-Bengal agar (0.5% peptone, 1% glucose, 0.1% KH2PO4, 0.05% MgSO4, 2% agar, 0.0033% bengal red, and 0.01% chloramphenicol; Overcast and Weakley, 1969) and incubated at 28°C for 48 h. Rose Bengal antimicrobic supplement for selective isolation and enumeration of yeast and molds from Jiulao. The representative of each colony morphotype was purified by repeated streaking on the Rose-Bengal agar plates and a total of 11 yeast strains were isolated from Daqu.
Ester production analysis After activation in YPD broth (1% yeast extract, 1% peptone, 2% glucose), the 11 yeast strains from Daqu were separately inoculated at an inoculation rate of 3% (v/v) into 80 mL of ester-producing medium (8% glucose, 1% yeast extract, and 2% peptone) in a 150-mL conical flask and incubated at 28°C for 7 days. Ester production was determined by the saponification method according to the Chinese National Standard GB 19777-2013 (Li et al., 2008). A commercial aroma-producing yeast strain (Thermal Resistance Alcohol Active Dry Yeast, Angel Yeast Co., Ltd., Yichang, Hubei, China) was used as the control.
Morphological, physiological and biochemical analyses The 11 yeast strains were preliminarily identified by their colony features and by their cellular, hyphal, and spore morphologies (Moemen, 2015). Y18 was selected due to its higher capability to produce esters than the control strain. The Y18 strain was streaked onto the malt extract solid medium (0.01% chloramphenicol, 13% malt extract, and 1.5% agar) and incubated at 25°C for 1 month. The cell shape, size, and budding were evaluated using a light microscope (CX21FS1, Olympus, Tokyo, Japan) at 100× magnification. Corn meal agar (0.2% corn meal extract, 1.5% agar) and spore-producing agar (1% KOAc, 2% agar, 0.1% yeast extract, and 0.05% glucose) were used to assess the formation of pseudohyphea and ascospores. The cell morphology was analyzed by the methylene blue method (Pfaller et al., 2004).
The physiological and biochemical characteristics of Y18 strain were determined in duplicate under aseptic conditions (Yarrow, 1998). For sugar assimilation test, the activated Y18 was inoculated into 10-mL tubes with an inverted Durham's fermentation tube and 3 mL of soybean sprout extract-glucose medium (12.5% soybean sprout extract and 2% sugar). Tubes were incubated at 28°C for 2–3 days before checking. The presence of air bubbles in the Durham's fermentation tube indicates that Y18 was able to assimilate the sugar. Six sugars were tested, including glucose, maltose, raffinose, lactose, galactose, and sucrose.
Assimilation of carbon and nitrogen sources was tested on MMA medium (1% glucose, 2% agar, 0.5% (NH4)2SO4, 0.01% KH2PO4, 0.01% MgSO4, and 0.01% vitamins), with different carbon (1%) or nitrogen (0.5%) sources substituting for glucose or (NH4)2SO4, respectively. Melibiose assimilation was tested in YPM broth (1% yeast extract, 1% peptone, and 2% melibiose). Plates and tubes were incubated at 30°C for 3 days before checking.
In addition, the activated Y18 was inoculated into litmus milk medium (10% skim milk powder and 0.0075% litmus, Tanasupawat et al., 1998) and incubated at 25–28°C for two to four weeks. The occurrence of milk coagulation, peptonization, or color change was considered positive for the litmus milk test. Amyloid synthesis test was performed in tubes containing PYG broth (2% peptone, 0.5% glucose, 1% yeast extract) with 1–2 drops Lugo's iodine and the results were checked after three weeks of incubation at 28°C. A blue, purple, or green color indicates that Y18 was able to produce amyloids. Urease test was conducted on a urea agar medium (0.1% enzymatic digest of gelatin, 0.1% dextrose, 0.5% sodium chloride, 0.2% monopotassium phosphate, 2% urea, 0.0012% phenol red, and 2% agar) and plates were incubated at 28°C for 4–5 days before checking. A red color means that Y18 was able to produce urease to break down urea.
pH, ethanol, glucose and heat tolerance tests The tolerance tests were carried out as previously reported (Visintin et al., 2016). All tests were carried out in duplicate in 10-mL tubes with a loading of 3 mL of YPD broth (1% yeast extract, 1% peptone, and 2% glucose) and cultured under semi-anaerobic conditions. For the pH tolerance test, the pH was adjusted to 1, 1.5, 2.0, 2.5, 3.0, and 4.0 with 1 M HCl. To test the tolerance for ethanol, ethanol was added to a final concentration of 6%, 8%, 10%, and 12% (v/v). Heat tolerance was tested using the medium (pH 5.5) at 30°C, 35°C, 40°C, 45°C, and 50°C. To test the tolerance for glucose, glucose was added to a final concentration of 10%, 20%, 30%, 40%, 50%, and 60% (w/v). All 10-mL tubes were inoculated with 1% (v/ v) of Y18 culture grown in YPD broth overnight at 28°C. Cell growth was assessed by determining the wet weight of the cell pellet after centrifugation at 4,000 × g for 10 min.
D1/D2 domain sequence analysis The genomic DNA of Y18 was extracted from an overnight culture in 9 mL of YPD broth (1×107−2×107 cells mL−1). The extracted DNA was purified using a Yeast Genomic DNA Extraction Kit (CW0569, ComWin Biotech Co. Ltd, Beijing, China) following the manufacturer's instructions. The D1/D2 domain of the 26S rDNA gene was amplified with primers synthesized by SBS Genetech Co. Ltd. (Beijing, China), i.e., NL1: 5′-GCA TAT CAA TAA GCG GAG GAA AAG-3′ and NL4: 5'-GGT CCG TGT TTC AAG ACG G-3' (Fliegerova et al., 2006). After 30 PCR cycles (94°C for 1 min, 52°C for 1 min, and 72°C for 50 s), the amplicons were purified and then sequenced by Invitrogen (Shanghai, China).
BLAST searches were performed using the NCBI GenBank data library (www.blast.ncbi.nlm.nih.gov/Blast). Sequence alignments of the D1/D2 domain were performed using Clustal X v1.83 (www.clustal.org/). Homologous sequences were obtained from GenBank (www.ncbi.nlm.nih.gov/nuccore/829582513/). A distance tree was generated using the BLAST pairwise alignments by the neighbor joining method in Mega 5.0 (www.megasoftware.net). The sequence of Y18 was deposited in Genbank with the accession number KP339953.1.
Y18-fortified alcoholic fermentation Y18 was sub-cultured for two generations in YPD broth at 30°C and then inoculated at 7% (v/v) into the Daqu-based fermentation broth in triplicate. Each fermentation broth was prepared with 80 g of dried sorghum flour (Shengxing, Shengxing Food Factory, China) that had been soaked in 96 mL of distilled water for 24 h (60% humidity) and steamed for 1.5 h under sterile conditions. The steamed sorghum flour was cooled to around 28°C before mixing with 20 g of Daqu powder, 300 U (0.28 g) of activated glucoamylase (G88275, Ruiyang Biotech. Co. Ltd., Wuxi, Jiangsu, China), 23 mL of activated Y18 culture (23 mL of distilled water for the control), and 144 mL of distilled water.
The mixture was transferred into 500-mL Erlenmeyer flasks capped with cotton plugs for alcoholic fermentation in a laminar flow cabinet at 25°C. The flasks were stirred using aseptic glass rods once a day for the first two days. Immediately after the second stirring (as before), all flasks were sealed with double layers of plastic film and allowed to ferment for another eight days. The alcoholic degree of alcoholic fermentation was measured using a vinometer (513, aiPLi, Quzhou, Zhejiang, China) after distillation (Wu et al., 2012). Analyses of the samples were conducted under sterile conditions. For the control, Daqu was the only starter without the inoculation of Y18.
Y18-fortified acetic acid fermentation The alcoholic fermentation broth of Y18 and control (363 mL each) were transferred into 1-L plastic buckets and solidified with dried sorghum flour, rice husk, and wheat bran at a mass ratio of 1:1.2:1.6 (1 portion of dried sorghum flour was 80 g). Vinegar seeds (∼6%, v/v) with acetic acid bacteria were inoculated into the buckets in the form of a fermenting matrix that was collected at the second day of acetic acid fermentation (Tongbao Vinegar Co., Ltd.). The buckets were covered with straw mats and incubated at 25°C From day 3 through 9, the matrix was aseptically mixed once a day by hands. NaCl (4 g) was added to terminate the fermentation, and 350 mL of distilled water was added and allowed to filter for 24 h. The vinegar was collected to measure the levels of total acids, non-volatile acids, total esters, reducing sugars, and amino nitrogen (Li et al., 2008).

A flow chart of vinegar fermentation in the present study
Aroma compound analysis The triplicate samples of alcoholic fermentation broth were collected, thoroughly mixed, and centrifuged at 2,400 × g for 15 min at 4°C. The supernatant (10 mL) with NaCl (3 g) dissolved in it was transferred into 20-mL headspace vials for gas chromatography-mass spectrometry (GC-MS) analysis. Each sample (2 mL) was transferred into another headspace vial with an autosampler (Thermo Fisher Scientific Inc., Waltham, MA, USA) and heated to 70°C for 30 min. The sample was analyzed using the GC-MS TRACE-ISQ (Thermo Fisher Scientific Inc.) equipped with a TR-5MS column (0.25 mm × 30 m, 0.25 µm; Thermo Fisher Scientific Inc.).
System control and data acquisition were performed using the Thermo Xcalibur 2.2 SP 1.48 software (Thermo Fisher Scientific Inc., USA). The sample (2 µL) was injected via the split-less mode at an injection temperature of 250°C. The flow rate of the carrier gas (He) was 1 mL/min. The column temperature was held at 35°C for 2 min, increased by 5°C min−1 to 230°C, and held at 230°C for 5 min. The transfer line and ion source temperatures were 280°C and 250°C, respectively. Ions were generated by electron ionization at 70 eV. Mass spectra were recorded at 5 scan s−1 over a mass range (m/z) of 30–350.
Peak detection and peak area calculation were performed using the Xcalibur software (Thermo Fisher Scientific Inc.). After the GC-MS analysis, identification of all volatile compounds was carried out by comparing their retention times with those of standard compounds and with the Nist 2011 mass spectral libraries. Peaks were annotated by comparing their unique mass spectra with those recorded in the National Institute of Standards and Wiley 7N Edition libraries (Wiley Registry of Mass Spectral Data, 7th Edition, 2000). The peak area was normalized against the 3-octanol internal standard and the relative abundance of aroma compounds was calculated as the percentage of total peak area on gas chromatogram.
Quality assessment and sensory analysis The quality of the vinegar was assessed by measuring the levels of five groups of basic compounds according to the Chinese National Standard (GB/T 19777-2013). Total acids and non-volatile acids were determined using the acid-base titration method (GB/T5009.41-2003 and GB18187-2000). Total eaters were determined by the saponification method (GB19777-2005). Reducing sugars were determined using Fehling's reagent (GB19777-2005). Amino nitrogen was determined using the formaldehyde titration method (GB/T5009.39-1996).
The vinegar was heated to 45°C and subjected to sensory analysis (Cejudo-Bastante et al., 2013) by a trained panel of seven women and six men with medium/high experience in sensory analysis of vinegars. The judges were provided with a group of macerated vinegar, representative of the complete set included in the study, and were asked to describe them qualitatively. The Y18-fortified vinegar was compared to the control and a commercial Shanxi aged-vinegar sample (Tongbao Vinegar Co., Ltd.). Prior to descriptive profiling, a stage for generating appropriate descriptors to define the samples was necessary. Quantification of seven analytical descriptors was carried out by using interval scales (International Organization for Standardization, 2003). The descriptors including ester, sour, intensity, fruity, flowery, sweet, and aftertaste were expressed as numerical values ranging from 0 to 100 and presented as averages from 13 panel members. The panel carried out two different tests.
Statistical analysis The results are expressed as mean ± standard deviation. Data were analyzed by one-way analysis of variance (ANOVA) at the 5% and 1% levels of significance. Statistical analysis was performed using the SPSS 16.0 Statistics (SPSS Inc., Chicago, USA).
Ester-producing capacity of yeasts The alcohol-producing yeasts accounted for the majority of fermenting microorganisms during alcoholic fermentation. A total of 19 representative yeast strains were obtained on the selective Rose-Bengal plates and labeled as Y1 to Y19. Eleven of these strains showed relatively high ester-producing capacity compared to the control strain (11.16 g L−1). Y18 had the third highest ester-producing capacity (33.48 g L−1) after Y2 and Y14, which was 3-fold as high as that of the control strain (Table 1). Based on the sensory analysis results, Y18 conferred fresh fruity, slightly sweet, moderate wine flavors, resulting in a product that was better than those by Y2 or Y14. Thus, Y18 was chosen for further analysis.
| Yeast strain | Ester production (g L−1)a | Significanceb | |
|---|---|---|---|
| 5% level | 1% level | ||
| Y2 | 33.76±0.14 | b | B |
| Y5 | 32.09±0.26 | d | D |
| Y6 | 29.67±0.47 | e | E |
| Y9 | 23.71±0.85 | i | I |
| Y10 | 28.25±0.64 | f | F |
| Y11 | 27.15±0.71 | g | G |
| Y12 | 32.90±0.19 | c | C |
| Y13 | 26.46±0.59 | h | H |
| Y14 | 36.06±0.48 | a | A |
| Y15 | 18.29±0.40 | j | J |
| Y18 | 33.48±0.22 | b | B |
| Control strainc | 11.16±0.29 | k | K |
Morphological and physiological characteristics of Y18 Following the culture in the solid malt extract medium, Y18 cells were ovoid or sausage-shaped, (4.0–7.2) × (3.0–5.2) µm in size. The vegetative cells appeared singly, in pairs, chains, or clusters (Fig. 2a). The colonies were slightly gray, with smooth surfaces and margins. No ascospores were observed, while pseudohyphae became visible at 2–4 days of culture (Fig. 2b). When stained by methylene blue, alive cells were able to produce enzyme that could reduce the blue dye into a colorless form; thus the alive cells were not dyed into blue, while the dead ones were blue (Fig. 2c).

Optical microphotographs showing the cell morphology of strain Y18 isolated from Daqu (a) vegetative cells (grown in solid malt extract medium, 400× magnification), (b) pseudohyphas (grown in corn meal agar, 100× magnification), and (c) methylene blue staining (grown in YPD broth, 100× magnification)
The ability of Y18 to assimilate glucose was considerably weak. In addition, Y18 could utilize a range of carbon and nitrogen sources, such as glycerol, D-mannitol, succinic acid, lactic acid, potassium nitrate, sodium nitrite, cadaverine, glucosamine, L-lysine, and ethylamine. The litmus milk test and amyloid synthetic test confirmed that Y18 could not utilize lactose. Urease test indicated that Y18 strain produce urease to break down urea (Table 2). The morphological and physiological analyses revealed that Y18 belongs to the Candida genus.
| Sugar assimilation | |||||||
|---|---|---|---|---|---|---|---|
| Glucose | + | Maltose | − | Raffinose | − | Lactose | − |
| Galactose | − | Sucrose | − | ||||
| Carbon source assimilation (as sole carbon source) | |||||||
| D-mannitol | + | Succinic acid | + | Lactic acid | + | Glycerol | + |
| Cellobiose | − | D-arabinose | − | Maltose | − | Ribitol | − |
| Trehalose | − | L-rhamnose | − | Raffinose | − | Inositol | − |
| D-gluconate | − | Butane-2,3-diol | − | Melibiose | − | Citric acid | − |
| D-xylose | − | Sucrose | − | D-Ribose | − | Salicin | − |
| Starch | − | Erythritol | − | Melezitose | − | Lactose | − |
| Propane-1,2-diol | − | Myo-inositol | − | L-sorbose | − | ||
| Nitrogen source assimilation(as sole nitrogen source) | |||||||
| Potassium nitrate | + | Sodium nitrite | + | Cadaverine | + | Glucosamine | + |
| L-lysine | + | Ethylamine | + | Creatinine | − | Imidazole | − |
| Other biochemical tests | |||||||
| Litmus milk test | − | Amyloid synthesis test | − | Urease test | + | ||
+, Positive; −, Negative
Growth characteristics of Y18 Y18 grew better at 40% (w/v) glucose than at 10%, 20%, or 30% (w/v) of glucose, with little growth at 50% or 60% (w/v) of glucose. Y18 proliferated at pH 2, but decreased sharply at pH 1.5. Y18 was capable of growing in the presence of ethanol up to 8%, while it was unable to grow beyond 12% ethanol. Y18 still grew well at 40°C, but not at > 40°C. The optimal growth conditions for Y18 was found to be at 30°C, pH 2, ethanol concentration of 8% (v/v), and glucose concentration of 40% (w/v). The critical parameters for vinegar fermentation of Y18 were determined to be 40°C, pH 2.5, 10% ethanol, and 40% glucose.
Molecular identification of Y18 The sequence analysis results revealed that the D1/D2 domain of the 26S rDNA gene in Y18 was 534 bp in length (Fig. 4). Based on the most often detected intraspecies sequence variability of 0–3 nucleotide differences in the D1/D2 LSU rRNA domain (Heide-Marie et al., 2009), Y18 was identified as Candida ethanolica. A neighbor-joining tree of Y18 was generated using the BLAST pairwise alignments (Fig. 5). The sequence of strain C. ethanolica Y18 was deposited in the China General Microbiological Culture Collection Center (CGMCC 2.5275, Beijing, China).
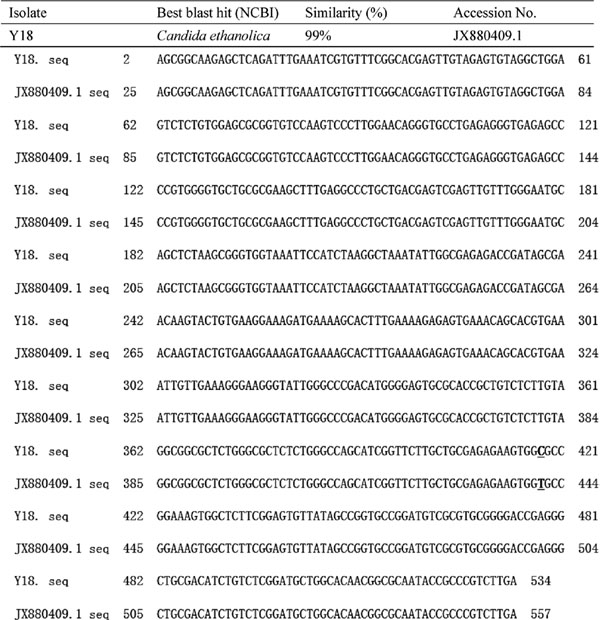
The sequence alignment of the D1/D2 domain of the 26S rRNA gene in strain Y18

Neighbor-joining tree based on 534 bp of the 26S rDNA gene sequences from Daqu-originated Candida ethanolica Y18. Bootstrap values shown at nodes for frequencies at or above a 50% threshold (1000 bootstrap resampling). Bar indicates 0.5% sequence variance
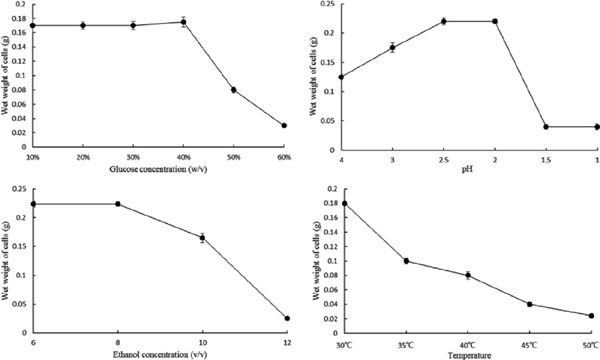
Growth characteristics of strainY18 as a function of (a) glucose concentration, (b) pH, (c) ethanol concentration, and (d) temperature. Data are presented as mean ± standard deviation of three replicates.
Alcoholic degree and aroma compounds in Y18-fortified alcoholic fermentation broth The alcoholic degree of the Y18-fortified alcoholic fermentation broth was markedly higher than the non-inoculated control (9.51%±0.04, v/v and 9.42%±0.06, v/v; p < 0.01). The major volatile compounds were roughly identical in the Y18-fortified alcoholic fermentation broth and the control (Fig. 6). Table 3 shows the difference in the aroma compounds produced by Y18-fortified alcoholic fermentation and the control. Twenty-eight aroma compounds in the control consisted of 60.74% alcohols, 12.94% esters, 0.64% aldehydes, and 25.68% other compounds such as 2-pentylfuran and non-identified components. In Y18-fortified fermentation broth, a total of 33 aroma components were detected by GC-MS, and the relative abundances of total alcohols, esters and aldehydes increased to 61.87%, 18.33%, and 0.73%, respectively. In contrast, the relative abundance of non-identified components decreased to 19.07%.
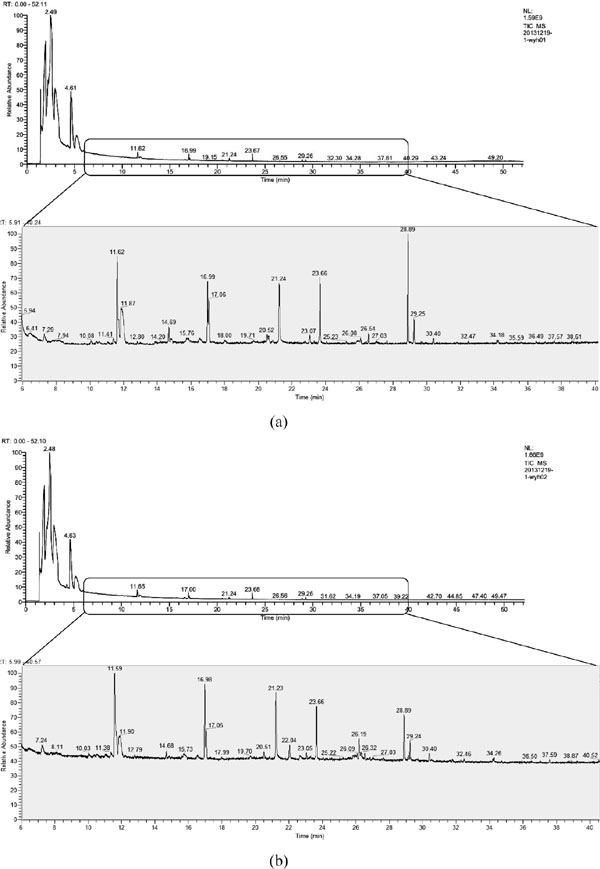
GC-MS ion chromatograms (0–50 min and 6–40 min) of volatile compounds in the Daqu-based alcoholic fermentation broth (a) Candida ethanolica Y18-fortified; and (b) non-inoculated control.
| Type | Daqu RTc (min) | Y18 RT (min) | Compounda | Aroma feature | Percent peak area (%) | |
|---|---|---|---|---|---|---|
| Control | Y18-fortified | |||||
| Alcohols | 2.56 | 2.51 | Ethanol | Spirit flavor and piquant | 31.91 | 38.00 |
| 5.20 | 5.22 | Isopentanol | Apple-like, pungent | 28.51 | 23.48 | |
| 21.23 | 21.24 | Phenylethanol | Rosy | 0.26 | 0.25 | |
| 5.62 | 5.63 | 2-Methyl-1-butanol | Piquant and irritate | 0.03 | 0.02 | |
| 11.38 | 11.41 | Hexanol | Fruity fragrance | 0.03 | 0.02 | |
| 22.79 | Isoborneol | Camphor-like smell | NDb | 0.01 | ||
| 6.41 | Isobutanol | Liquor flavor and piquant | ND | 0.09 | ||
| Subtotal | 60.74 | 61.87 | ||||
| Esters | 2.95 | 2.96 | Ethyl acetate | Strong ether smell; clear and slight fruity, liquor fragrance | 9.84 | 14.46 |
| 11.59 | 11.62 | Isoamyl acetate | Strong fresh fruity aroma | 1.03 | 1.52 | |
| 16.98 | 16.99 | Ethyl caproate | Flavor of pineapple and banana | 0.68 | 0.78 | |
| 17.05 | 17.06 | Ethyl octanoate | Rosy, aroma of orange flower and fruit | 0.59 | 0.62 | |
| 29.24 | 29.25 | Ethyl caprate | Wine fragrance | 0.21 | 0.22 | |
| 7.24 | 7.29 | Ethyl butyrate | Heavy, clear and elegant fragrance of sweet fruit, pineapple, banana and apple | 0.20 | 0.15 | |
| 5.91 | 5.94 | Isobutyl acetate | Smell of ripen fruit | 0.11 | 0.05 | |
| 28.89 | 28.89 | Isobornyl acrylate | 0.08 | 0.13 | ||
| 20.51 | 20.52 | Ethyl heptanoate | Smell of fruit and wine, pleasant burnt taste | 0.07 | 0.09 | |
| 10.03 | 10.08 | Ethyl | Strong aroma of peels of apple, pineapple and immature plum | 0.03 | 0.12 | |
| 2-methyl, butyrate | ||||||
| 23.05 | 23.07 | Ethyl benzoate | Slight fruit flavor | 0.03 | 0.03 | |
| 11.90 | 11.87 | Isovaleric acid, ethyl ester | Apple like and mulberry like flavor | 0.02 | 0.05 | |
| 25.22 | 25.23 | Isopentyl hexanoate | Fragrance of apple and pineapple | 0.02 | 0.01 | |
| 38.87 | 38.61 | Ethyl palmitate | Weak fragrance of wax and butter fat | 0.02 | 0.02 | |
| 34.26 | 34.18 | Ethyl laurate | Peanut fragrance | 0.01 | 0.02 | |
| 20.44 | Benzoic acid, methyl ester | Strong flowery and fruity aroma, powerful wintergreen oil taste | ND | 0.02 | ||
| 25.60 | Acetic acid, 2-phenylethyl ester | Honey and rose fragrance | ND | 0.02 | ||
| 14.20 | Hexanoic acid, phenyl ester | ND | 0.02 | |||
| Subtotal | 12.94 | 18.33 | ||||
| Aldehydes | 4.29 | 4.26 | Acetal | Flowery, rosy, honey-like fragrance | 0.51 | 0.64 |
| 15.73 | 15.76 | Benzaldehyde | Special almond fragrance | 0.03 | 0.02 | |
| 17.99 | 18.00 | Octanal | Fruity | 0.01 | 0.01 | |
| 19.70 | 19.71 | Nonanal | Rosy, flavor of oranges and tangerines, strong lipid taste | 0.03 | 0.02 | |
| 27.03 | 27.03 | Undecanal | Rosy, waxy and fatty smell, sweet tangerine taste | 0.02 | 0.01 | |
| 30.40 | 30.40 | Lauraldehyde | Violet fragrance at low concentration | 0.02 | 0.01 | |
| 26.09 | 26.08 | Decanal | Sweet, orange and tangerine like, waxy, flowery | 0.02 | 0.02 | |
| Subtotal | 0.64 | 0.73 | ||||
| Others | 14.68 | 14.69 | 2-Pentylfuran | Fruity aroma | 0.17 | 0.21 |
| Non-identified | 25.51 | 18.86 | ||||
| Subtotal | 25.68 | 19.07 | ||||
| Total | 100 | 100 | ||||
GC-MS data showed that ethanol comprised a large percentage (>30%) of the total peak area at the retention time of ∼2.5 min for both the Y18-fortified fermentation broth and the control.
Even though the relative abundance of isopentanol decreased by 5.03% in the Y18-fortified fermentation broth, the relative abundance of ethanol markedly increased by 6.09% compared to the control. In addition, the relative abundance of ethyl acetate increased by 4.62% compared to the control. Despite the relative decrease of a few esters (isobutyl acetate and isopentyl hexanoate), other esters (ethyl acetate, isoamyl acetate, ethyl caproate, and ethyl octanoate) increased to varying degrees. The relative abundance of aldehydes was < 1% and this type of compounds contributed to seven fruity flavors. Five compounds including isoborneol ethanol, isobutanol, acetic acid 2-phenylethyl ester, hexanoic acid phenyl ester, and benzoic acid methyl ester were detected in the Y18-fortified fermentation broth, but not in the control. Benzoic acid methyl ester has a flowery, cherry-like smell and a powerful wintergreen oil taste (Table 3).
Quality and sensory characteristics of the Y18-fermented vinegar The levels of both total acids and total esters were significantly higher in the Y18-fermented vinegar compared to the control (p < 0.01). Y18 did not significantly affect the levels of non-volatile acids, reducing sugars, or amino nitrogen in the vinegar as compared to the control (Table 4).
| Vinegar | Total acids | Non-volatile acids | Total esters | Reducing sugars | Amino nitrogen |
|---|---|---|---|---|---|
| Control | 37.42±0.31b | 8.96±0.64 | 15.30±0.64b | 13.03±0.68 | 15.28±0.42 |
| Y18-fortified | 40.24±0.43a | 9.03±0.29 | 19.25±0.87a | 13.33±1.12 | 15.19±0.27 |
The sensory analysis showed that all vinegars were tested as sour. Compared to the control and the commercial Shanxi aged-vinegar, the Y18-fortified vinegar had higher sour, fruity, and flowery scores (Table 5). It is worth noting that the commercial vinegar has been ripened for at least one year, while the Y18-fortified vinegar was only fermented for one month. It would have been expected that the Y18-fortified vinegar could obtain more superior quality should it be fermented for a year; however this is not within the scope of the current study and future work is needed to verify the hypothesis.
| Vinegara | Ester | Sour | Intensity | Fruity | Flowery | Sweet | Aftertaste |
|---|---|---|---|---|---|---|---|
| Control | 20 | 42 | 20 | 2 | 2 | 13 | 20 |
| Y18-fortified | 32 | 45 | 18 | 12 | 8 | 12 | 26 |
| Commercial Shanxi aged-vinegar | 35 | 42 | 15 | 2 | 2 | 15 | 30 |
Data are average scores (0–100) from 13 panel members.
The present study was performed under strict control to ensure that all the isolated yeast strains were originating from Daqu. The sequence of the D1/D2 domain of the 26S rRNA gene was used in combination with the phenotypic traits for the identification of strain Y18 with high ester-producing capacity. The results revealed that Y18 was a yeast strain of Candida ethanolica. Members of Candida have been found in Daqu (Wu, 2004). Unlike several Candida species, C. ethanolica cannot assimilate nitrate, ferment sugars, or produce urease (Nguyen et al., 2011). The results from the current study supported these conclusions for the isolated Y18 strain. In addition, a species similar to C. ethanolica was isolated from the sour cassava starch in Brazil (Lacerda et al., 2005). C. ethanolica was reported to increase the growth yield of tomato (Nguyen et al., 2011).
Saccharomyces cerevisiae is the most sensitive yeast species to low pH values, limiting its application in the fermentation of Shanxi aged-vinegar (Heide-Marie et al., 2009). In the present study, C. ethanolica Y18 was found to proliferate at pH 2.0. Y18 was isolated from the Jiulao 144 h after the start of alcoholic fermentation. The growth tests revealed that C. ethanolica Y18 could survive throughout the multiple stages of vinegar fermentation both in the lab-scale and large-scale industrial applications. In the early stage of acetic acid fermentation following alcoholic fermentation, Y18 still survived and produced esters. Nevertheless, C. ethanolica can be destroyed with the addition of table salt or by pasteurization.
Spiking Daqu with the Daqu-originated Y18 markedly increased the relative abundances of ethanol in Jiulao. As an aroma-producing yeast, Y18 has considerably weak capacity for assimilation of sugar. The possible reason for the increase of ethanol is that the proliferation and metabolism of Y18 at the early stage of alcoholic fermentation created a favorable circumstance for the activity of alcohol-producing yeasts in Daqu. The addition of Y18 increased the production of esters, especially ethyl acetate, which is the most prevalent aroma compound in the traditional fermented liquors and vinegars (Wang et al., 2015; Ubeda et al., 2011). The increase of ethanol is beneficial to the formation of acetic acid (by ethanol oxidation) and ester. For example, ethyl acetate is formed by the esterification between acetic acid and ethanol during alcoholic fermentation and acetic acid fermentation. Y18 also decreased the relative abundance of isopentanol (pungent taste), which contributed directly to a relative increase of isoamyl acetate (strong fresh fruity and flowery aroma).
The majority of the aroma compounds (isopentanol, isoamyl acetate, and ethyl caproate) present in the Y18-fortified fermentation broth imparted flowery and/or fruity flavors. These results were similar to those obtained by Cao et al., (2015) where they reported the increased aroma compounds of ethyl acetate, isopentanol, and isoamyl acetate with the inoculation of Saccharomyces cerevisiae. Isoamyl acetate is an important flavor compound used in the food industry (Krishna et al., 2001) and it is involved in the aroma and taste of several fermented foods (Ubeda et al., 2011; Cao et al., 2015; Krishna et al., 2001). Despite its extremely low level in brandy, isoamyl acetate improves the aroma and quality of the liquor.
The sensory analysis confirmed that the vinegar fermented with Y18-fortified Daqu as starter provided excellent quality, fresh fruity and floral notes, and a long-lingering buttery and nut-like taste. There were no negative attributes in the vinegar. The results are in agreement with the findings of Inoue et al., (2013), who used C. ethanolica, L. casei, and L. rhamnosusobtain as starter for the production of liquefied fermented broth (Inoue et al., 2013).
In conclusion, the inoculation of C. ethanolica Y18 into the Daqu-based fermentation broth increased the contents of total acids and total esters, and enhanced the aroma of Shanxi aged-vinegar in terms of the pleasant fruity and flowery flavors and better aftertaste.
Acknowledgements The study was funded by the Key Project of Science and Technology of Shanxi Province, China (2015-TN-10-2) and the Key Project of Shanxi Key R&D Program of China (201703D211001-06-05).
Author 1 declares that she has no conflict of interest.
Author 2 declares that she has no conflict of interest.
Author 3 declares that she has no conflict of interest.
Author 4 declares that he has no conflict of interest.