2018 年 24 巻 6 号 p. 1083-1092
2018 年 24 巻 6 号 p. 1083-1092
The best way to prepare glycated chicken myofibrillar proteins (Mfs) with the strongest antioxidative ability against hydroxyl radicals (•OH), without decreasing solubility in a low ion strength medium, was identified using random-centroid optimization (RCO). Four factors (temperature, relative humidity (RH), reaction time and quantity of ribose) were selected, and 13 vertices were obtained using RCO. Evaluations were carried out relative to each vertex, and the optimal preparatory conditions were sought. Optimal conditions were identified as: temperature, 38°C; RH, 40%; reaction time, 4.55 h; and ribose weight ratio, 10.7. The •OH antioxidant capacity was approximately 5.1 ± 1.3 µmol of gallic acid equivalent/g of protein. Under these conditions, glycated chicken Mfs lost their thermal gel-formation activity.
In previous studies, we found that the glycation of chicken myofibrillar proteins (Mfs) with maltose via the Maillard reaction, induced functional alterations, such as improved solubility in a low ionic strength medium, higher thermal stability and antioxidant abilities (Nishimura et al., 2011a,b). The superoxide anion radical scavenging activity (SOSA) value of the obtained glycated chicken Mfs was 184 ± 40 units (U) of superoxide dismutase (SOD)/g of protein. This antioxidant ability is attributed to the primary structure, the specific peptide sequence of the glycated chicken Mfs, irrespective of the protein shape (Isono et al., 2012). Moreover, it was also found that these glycated chicken Mfs have thermal gel-forming activity, and thus it was considered that this thermal gel might retain antioxidant activity (Nishimura et al., 2015). To evaluate the antioxidant properties of a thermal gel formed from the glycated chicken Mfs, hydroxyl radical antioxidant capacity (HORAC), instead of SOSA, was used as an index of antioxidant activity, as SOSA, in the context of a solid sample, cannot be determined by electron spin resonance. The conditions to achieve the strongest antioxidant ability against hydroxyl radicals (•OH) with a solubility of more than 60% in a low ionic strength medium (Nishimura and Saeki, 2016) were designed using random-centroid optimization (RCO) (Nishimura et al., 1997, 1998; Goto et al., 2000; Nishimura et al., 2001; Goto et al., 2004; Nakai et al., 2009; Yan and Wang, 2015; Nishimura and Saeki, 2016, 2018). Optimal conditions of 57°C, 37% relative humidity (RH), 37.2 h reaction time, and a maltose mixing ratio of 5.43 (w/w) were identified. The HORAC value of the glycated chicken Mfs with maltose prepared under optimal conditions was 7.8 ± 1.0 µmol of gallic acid (GA) equivalent/g of protein, this value was comparable to those for apple and white peach extracts (15.0 and 23.1 µmol of GA equivalent/g of protein, respectively) (Boxin et al., 2002). Furthermore, the thermal gel that formed after 30 min of heating at 90°C retained antioxidative activities, although HORAC values were reduced to 4.4 ± 1.7 µmol of GA equivalent/g of protein.
Generally, processed meat products requiring gel-formation of Mfs, such as sausages and boiled fish pastes, need large quantities of salt to dissolve Mfs during production, and the addition of an antioxidant is necessary for product storage. It is known that the salt is one of the risk factors causing lifestyle-related diseases such as high blood pressure, and that consumers avoid buying processed foods containing additives because of health concerns. The application of glycated chicken Mfs to processed meat products could contribute to reducing levels of food ingredients such as salt and antioxidants, suggesting that processed meat products could become healthier.
However, using maltose for glycation required 37.2 h for the Maillard reaction to proceed and 2 days of dialysis was required to remove unreacted maltose, suggesting that maltose use is not practical as a sugar for protein-glycation. Therefore, the authors investigated monosaccharides, which can undergo chemical reactions more easily, affecting food functionality (Nishimura and Saeki, 2018). Among monosaccharides, ribose, which has a five-member ring that should react faster than a six-member ring, was selected. SOSA values, as an index of antioxidant capability, were used for comparison with antioxidant abilities obtained in other studies (Nishimura et al., 2011a,b; Isono et al., 2012). Chicken Mfs glycated with ribose prepared under optimal conditions (38°C, 39% RH, 3.08 h reaction time, and 10.4 ribose mixing ratio (w/w)), which were found using RCO, showed a SOSA value of 368 ± 120 U of SOD/g of protein. The SOSA value of the glycated chicken Mfs with maltose prepared under the optimal conditions (61°C, 38% RH, 33.9 h reaction time, and 5.59 maltose mixing ratio (w/w)), was 274 ± 86 U of SOD/g of protein. There were no significant differences between both SOSA values, suggesting that similar antioxidant activity can be developed using ribose instead of maltose. Moreover, the reaction time using ribose was truncated by approximately 30 h, and the reaction temperature was lowered by approximately 20°C, indicating that using ribose was efficient when preparing glycated chicken Mfs. If glycated chicken Mfs with ribose have thermal gel-forming activity, we would be able to use these proteins as material for processed meat products requiring gel-formation of Mfs. In this study, both the thermal gel-forming ability of the glycated chicken Mfs with ribose and the antioxidant ability were investigated. Antioxidant ability was evaluated using HORAC, because in the context of a solid sample, SOSA cannot be determined by electron spin resonance, as described above.
Materials and chemicals Chicken breast meat was purchased from a local poultry farm immediately after slaughter. Radical Catch, a kit for •OH measurement, was purchased from Hitachi, Ltd. (Tokyo, Japan). GA was obtained from ChromaDex, Inc. (Irvine, USA). All other chemicals were of reagent grade and were obtained from Nacalai Tesque, Inc. (Kyoto, Japan) or Wako Pure Chemicals Industries, Ltd. (Osaka, Japan).
Preparation of myofibrillar proteins (Mfs) Chicken Mfs were prepared as described by Saeki (1997) with modification. Chicken breast meat was cut finely with a knife and suspended in 10 volumes of 0.1 M sodium phosphate buffer (pH 7.5). The solution was allowed to settle and the supernatant was decanted as waste. This step was repeated five times. Meat was then homogenized in a homogenizer (model AM-9; Nissei Co. Ltd., Tokyo, Japan) in 10 volumes (based on the initial muscle weight) of 0.1 M sodium phosphate buffer (pH 7.5) for 0.5 min at 10,000 rpm. This step was repeated four times. After passing through cotton gauze, 20% Triton X-100 solution was added to the filtrate to obtain a final concentration of 0.5% and then stirred gently for 3 min. After standing for 10 min, the filtrate was centrifuged at 7,000 × g for 10 min to collect Mfs. The precipitate was resuspended in 50 mM NaCl and was centrifuged at 7,000 × g for 10 min. This procedure was performed five times. The obtained precipitate was suspended in 100 mL of 50 mM NaCl with a hand homogenizer. Purified chicken Mfs were obtained by filtering the solution through cotton gauze. A portion of this fraction was dissolved in 0.5 M NaCl-15 mM sodium phosphate buffer (pH 7.5) for use as the native chicken Mfs solution. All preparation steps were carried out on ice.
Random-centroid optimization (RCO) RCO (Nishimura et al., 1997, 1998; Goto et al., 2000; Nishimura et al., 2001; Goto et al., 2004; Nakai et al., 2009; Yan and Wang, 2015; Nishimura and Saeki, 2016, 2018) was used to determine the optimal preparative conditions for glycated chicken Mfs, meeting the requirements of greater than 60% solubility in a low ionic strength medium and the strongest antioxidant ability. Briefly, glycated chicken Mfs were prepared relative to 13 vertices, which are a series of experimental conditions, dictating the four preparative condition parameters. Among glycated chicken Mfs, those with less than 60% solubility in a low ionic strength medium were evaluated as 100% in residual ratio of oOH. Accordingly, the evaluation of glycated chicken Mfs with greater than 60% solubility in a low ionic strength medium gave values below 100% because of the presence of antioxidative capacity. Vertices were evaluated to determine the smallest residual ratio of •OH. Each vertex was assessed independently in triplicate (more in some cases). Experimental ranges of each factor were defined as follows: temperature was 30–45°C, RH was 35–45%, reaction time was 1–6 h, and ribose mixing ratio (w/w) was 8–14.
Glycation of chicken Mfs The chicken Mfs were glycated according to the method of Saeki (1997), with some modification. Chicken Mfs, suspended in 50 mM NaCl, were mixed with ribose at weight ratios determined by the RCO vertex. After adjusting the final protein concentration to 6.0 mg/ mL, 5 mL of each Mfs-ribose solution was transferred to a test tube (16-mm diameter), frozen at −80°C, and immediately lyophilized in a freeze-dryer (FDU-1110; Tokyo Rikakikai Co., Ltd., Tokyo, Japan). This process destroyed the sarcomeres and exposed the Mfs. Lyophilization was terminated when the sample temperature reached 15–18°C. Each lyophilized protein powder was immediately stored at −40°C and used within 30 days of preparation. The Maillard reaction was conducted by incubating the lyophilized powders under the 13 conditions defined by the RCO. An incubator/humidity cabinet (KCL-2000A; Tokyo Rikakikai Co., Ltd., Tokyo, Japan) was used to control the temperature and RH.
Solubility of glycated chicken Mfs Solubility of the glycated Mfs was measured according to the method of Saeki and Inoue (1997), with some modification. After glycation, protein powder was immediately mixed with 0.1 M NaCl-15 mM sodium phosphate buffer (pH 7.5) in a T 10 basic ULTRA-TURRAX high-speed blender (IKA-Labotechnik, Staufen, Germany) at 13,500 rpm for 0.5 min, and this step was repeated again, with a final protein concentration of 1.5 mg/ mL. Homogenization was followed by centrifugation at 32,000 × g for 30 min at 4°C. The amounts of protein before centrifugation and in the supernatant were determined by the Kjeldahl method (AOAC, 1990). Total soluble Mfs were expressed as the percent protein concentration in the supernatant with respect to total protein before centrifugation.
Preparation of sample for measurement of •OH averting capacity (HORAC) After incubating for glycation, each protein powder was immediately stirred with 0.1 M NaCl-15 mM sodium phosphate buffer (pH 7.5) and dialyzed in the same solution at 8–12°C for a day to remove the unreacted ribose. After centrifugation at 32,000 × g for 30 min at 4°C, the supernatant containing 7–9 mg of protein/mL was used as the sample solution.
Measurement of HORAC HORAC was measured using an antioxidant potential measurement kit (Radical Catch; Aloka, Co., Ltd., Tokyo, Japan) and a chemiluminescence reader (AccuFLEX Lumi400, Aloka Co., Ltd., Tokyo, Japan) based on the Fenton reaction, in which antioxidants are trapped by luminol, resulting in light emission (Parejo et al., 2000). This is a procedure for assessing the •OH scavenging activity using ferrous iron-induced luminol chemiluminescence (Yildiz and Demiryürek, 1998). Aliquots of sample solution described above were harvested for measuring the antioxidant capacity. First, 50 µL of cobalt solution and 50 µL of luminol solution were mixed with 20 µL of diluted sample solution, which was adjusted to a final concentration of 0.59 mg protein/mL, and incubated for 5 min at 37°C. The •OH generation was initiated by addition of 50 µL of H2O2 solution. Light emission at 430 nm was measured for 120 s immediately after initiation. The control contained 0.1 M NaCl-15 mM sodium phosphate buffer (pH 7.5). Light emissions from 80 to 120 s were integrated. Among 13 samples, the residual ratio of •OH was calculated for samples that showed a solubility greater than 60% in low ionic strength medium. Antioxidant activity was measured as the relative light unit (RLU) of a sample/RLU of the control × 100. These values were used to evaluate RCO.
Determination of available lysine Assay of available lysine was carried out to evaluate the progress of the Maillard reaction between the Mfs and ribose. Optimized glycated Mfs were dissolved in 0.5 M NaCl-15 mM sodium phosphate buffer (pH 7.5) in a T 10 basic ULTRA-TURRAX high-speed blender (IKA-Labotechnik, Staufen, Germany), then precipitated with 7.5% trichloroacetic acid (final concentration) in iced water for 30 min. After removing the sodium phosphate buffer, unreacted sugars, and trichloroacetic acid by decantation, the precipitated protein was redissolved in a 50 mM sodium phosphate buffer (pH 9.5) containing 2% sodium dodecyl sulfate (SDS). The available lysine content was determined by spectrophotometric analysis using o-phthalaldehyde and N-acetyl-L-cysteine (Hernandez and Alvarez-Coque, 1992).
Formation of thermal gel A solution of optimized glycated chicken Mfs, which had the smallest residual ratio of •OH among the 13 sample solutions obtained as described above, was concentrated to 15 from 7–9 mg protein/mL in 0.1 M NaCl-15 mM sodium phosphate buffer (pH 7.5) as follows. i) Amicon Ultra-15 Centrifugal Filter Devices (Merck Millipore Co., Darmstadt, Germany) with centrifugation at 4,500 × g and 4°C, ii) burying tube for dialysis filled with solution in solid polyethylene glycol particles at 4°C, then 0.5 mL of the concentrated solution was placed in a test tube (16-mm diameter), sealed with polyvinylidene chloride film, and heated in a water bath at 90°C for 4 h. The sample was examined by SDS-polyacrylamide gel electrophoresis (PAGE) analysis. The antioxidant ability of the gel was determined by HORAC.
SDS-PAGE analysis of heat-induced gels Heated solutions were mixed with equal volumes of 125 mM Tris-HCl (pH 6.8) containing 8 M urea, 4% SDS, and 40 mM N-ethylmaleimide. Mixtures were allowed to stand overnight at room temperature. 2-Mercaptoethanol (2-ME) was added to each mixture to a final concentration of 10% (v/v), and the mixture was boiled for 3 min. Electrophoresis was carried out on a 7.5% acrylamide slab gel according to Laemmli's method (Laemmli, 1970), followed by staining with Coomassie Brilliant Blue R-250.
Evaluation of HORAC of GA and optimized glycated chicken Mfs The HORACs of GA and optimized glycated chicken Mfs, which indicated the smallest residual ratio of •OH, were calculated as follows. •OH was generated by the Fenton reaction. The RLU value containing no sample was regarded as 100%. Comparative RLUs in the presence of GA and optimized glycated chicken Mfs were calculated, and the relationship between RLU and the concentration of the chemical or optimized glycated Mfs was graphed. The amounts of GA and glycated chicken Mfs that elicited 50% RLU were determined as the half-maximal inhibitory concentration (IC50) values of GA and optimized glycated chicken Mfs, respectively. The IC50 value of an optimized glycated chicken Mfs solution (mg/mL) was converted to HORAC (GA equivalent/g of protein).
Protein determination The protein concentrations of dissolved chicken Mfs were determined as described by the Kjeldahl method (AOAC, 1990). Purified chicken Mfs and glycated chicken Mfs when measuring the amount of available lysine were determined using the Lowry method (Lowry et al., 1951), with bovine serum albumin as a standard. The biuret method (Gornall et al., 1949), which uses bovine serum albumin as a standard, was used in other assays.
Statistical analysis Each experiment was performed on three different lots of glycated Mfs. Results are reported as mean values of at least three determinations with the error bars indicating standard deviation. Statistical analysis was performed using Microsoft Excel Ver. 2013 with Ekuseru-Toukei 2010 (Social Survey Research Information Co., Tokyo, Japan). P < 0.05 was considered significant.
Determination of the optimal preparative method for glycated chicken Mfs with ribose RCO was used to determine the optimal conditions for production of ribose-conjugated chicken Mfs with the strongest antioxidant ability and greater than 60% solubility in a low ionic strength medium, by varying temperature, RH, reaction time, and ribose mixing ratio (w/w) (Table 1). The parameters in each experiment were calculated using the RCO program, and were then implemented for different preparations of glycated chicken Mfs. All data were mapped (Fig. 1), aiding the visualization of the experimental response surface and indicating the data trends (Nakai et al., 2009). Although RCO is usually repeated until an adequate response is achieved, in this study, the approximate position of the optimal conditions was clear after the first cycle of the RCO program. The best results were obtained near Vertex 13 with a temperature of 38°C, an RH of 40%, a reaction time of 4.55 h, and a ribose mixing ratio (w/w) of 10.7 (Fig. 1).
| Vertex | Temperature | RH | Reaction Time | Ribose Mixing Ratio | Evaluation (Residual ratio of •OH)a) |
|---|---|---|---|---|---|
| No. | (°C) | (%) | (h) | (w/w) | (%) |
| 1 | 35 | 40 | 4.85 | 11.7 | 65.5 |
| 2 | 39 | 37 | 1.15 | 8.2 | 73.8 |
| 3 | 39 | 43 | 4.92 | 11.0 | 100.0 |
| 4 | 43 | 44 | 3.60 | 10.1 | 100.0 |
| 5 | 44 | 38 | 1.22 | 9.7 | 100.0 |
| 6 | 30 | 43 | 3.37 | 11.0 | 100.0 |
| 7 | 43 | 44 | 3.25 | 13.0 | 100.0 |
| 8 | 35 | 37 | 5.95 | 8.7 | 100.0 |
| 9 | 39 | 39 | 4.17 | 11.2 | 100.0 |
| 10b) | 37 | 38 | 4.03 | 10.0 | 77.4 |
| 11b) | 38 | 40 | 3.35 | 11.0 | 71.2 |
| 12b) | 38 | 39 | 3.80 | 10.4 | 63.0 |
| 13b) | 38 | 40 | 4.55 | 11.2 | 58.7 |
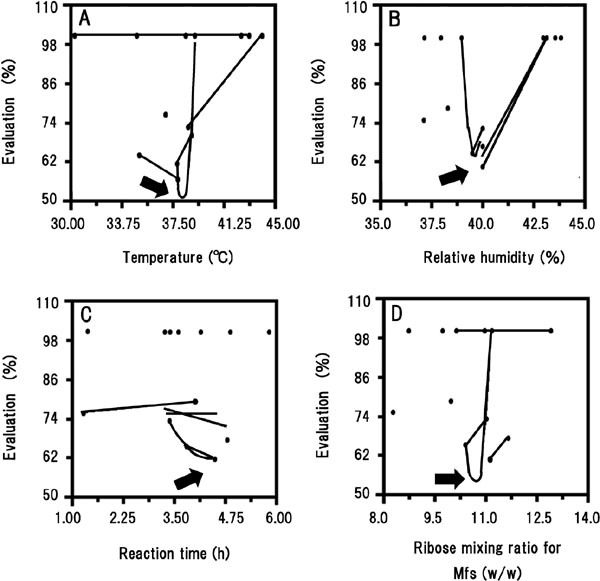
Mapping results of RCO derived experiments for scavenging •OH.
Evaluation: When the solubility in a low ionic strength medium of glycated Mfs did not exceed 60%, the evaluation of this vertex was estimated as 100%. The emitting intensity of •OH assessed by the Fenton reaction, detected with fluorescence spectroscopy, was regarded as 100%. The comparative emitting intensity of •OH, assessed by the Fenton reaction, in the presence of glycated Mfs was used for the evaluation (residual ratio of •OH) of antioxidative activity. The vertex that provided the smallest evaluation was sought. (A) temperature. (B) relative humidity. (C) reaction time. (D) ribose mixing ratio for Mfs (w/w). (●) was each vertex. Lines indicate probable trends. Arrow in each graph shows the best result.
When SOSA was employed as the index of antioxidant capability, the optimal condition was indicated as a temperature of 38°C, RH of 39%, a reaction time of 3.80 h, and a ribose mixing ratio (w/w) of 10.4 (Nishimura and Saeki, 2018). When comparing the results in Fig. 1 with the SOSA, temperature, RH, and the ribose mixing ratio (w/w) were similar, but the reaction time was slightly longer. This subtle difference might be due to the production yields of the intermediate and final Maillard reaction products with specific scavenging activity for the superoxide anion radical or •OH.
Time-dependent solubility and amount of available lysine in optimized glycated chicken Mfs When temperature, RH, and the ribose mixing ratio for Mfs (w/w) were fixed at 38°C, 40% RH, and 10.7, respectively, the time-dependent solubility of the optimized glycated chicken Mfs in 0.1 and 0.5 M NaCl, and the amounts of available lysine during the Maillard reaction were followed up to 4.55 h (Fig. 2). The solubility of the optimized glycated chicken Mfs in 0.5 M NaCl was 81.5 ± 10.4% (n = 3) (Fig. 2A), which was higher than the value obtained from maltose-conjugated chicken Mfs (55.1 ± 5.8% (n = 3)) (Nishimura and Saeki, 2016); this value increased to a maximum of 85.2 ± 6.2% (n = 3) 1 h later. Afterwards, solubility decreased gradually to 66.5 ± 8.5% (n = 3) by 4.55 h. The decline in solubility from 2 to 4.55 h may depend on the denaturation of some chicken Mfs. Meanwhile, the solubility in 0.1 M NaCl began at 42.4 ± 16.6% (n = 6) and increased to 78.0 ± 8.0% (n = 3) after a 2 h-incubation. Then, the value decreased continuously to 62.0 ± 3.3% at 4.55 h.

Changes in the solubility and quantity of available lysine in Mfs during the Maillard reaction with ribose.
The lyophilized chicken Mfs-ribose [Mfs:ribose = 1:10.7(w/w)] reaction was performed at 38°C and 40% RH for 4.55h. The solubility of optimized glycated chicken Mfs (A) in 0.1 (△) or 0.5 M (■) NaCl solution (pH 7.5) was measured. Simultaneously, changes in the amounts of available lysine (B) in the glycated protein were measured during the Maillard reaction. Values represent the mean ± standard deviation (n≥3).
Reflecting the solubility in 0.1 M NaCl, the available lysine content was not 100% but 52.4 ± 2.8% (n = 3) before incubation (Fig. 2B), suggesting that the Maillard reaction had already progressed during the pre-procedure. This phenomenon was likely due to the high reactivity of the five-membered ring structure of the ribose sugar. The amount of the available lysine decreased to 44.3 ± 2.8% (n = 3) by 0.5 h and subsequently declined to 30.4 ± 10.8% (n = 3) by 4.55 h.
Babij et al. (1991) reported that about 200 lysine residues were present in a molecule of the myosin heavy chain (MHC). Therefore, approximately 100 molecules of ribose were thought to be conjugated to Mfs at the outset of the reaction. One molecule of ribose has four hydroxyl groups, including a hydroxyl group connected to the anomeric carbon. The total number of hydroxyl groups in 100 molecules of ribose is 400 per molecule of the MHC, although the hydroxyl group connected with the anomeric carbon is used to react with lysine. One hydroxyl group is increased by ring-opening, resulting in a lack of variability of the total number of hydroxyl groups. Such conjugation resulted in increased hydration, which could also play a role in the improved solubility. A 0.5-h reaction resulted in solubility of over 60% in 0.1 M NaCl, suggesting that the conjugation of 440 of hydroxyl groups per molecule of MHC is a necessary condition to secure ≥ 60% solubility in 0.1 M NaCl. Maltose-conjugated chicken Mfs also showed water-solubility when an approximately 30% of available lysine decreased (Nishimura et al., 2011b; Isono et al., 2012), which means that the bonding of 400 of hydroxyl group was reacted with maltose. These data suggest that the connection of over 400 of hydroxyl groups in one myosin molecule results in solubility in a low strength medium ≥ 60%, irrespective of sugar used. However, solubility in a low strength medium was affected by not only the quantity of hydroxyl groups, but also other factors (Katayama et al., 2002). Therefore, the complex result may be derived from interactions between several factors.
Thermal gel-forming abilities of optimized glycated chicken Mfs Optimized glycated, and unmodified chicken Mfs solutions (15 mg of protein/mL) were heated at 90°C for 240 min, and subsequent gel formation was observed (Fig. 3). The turbidity of the unmodified chicken Mfs solution (Fig. 3A) increased with heating, but no changes were observed in the color of the solution over time. Furthermore, the solution did not solidify with heating, as observed upon tilting the test tube. Previous studies (Nishimura et al., 2015: Nishimura and Saeki, 2016) with similar observations indicated that the absence of sugar caused denaturation of protein during incubation, leading to the loss of gel-forming activity. The optimized glycated chicken Mfs solution (Fig. 3B) became turbid upon heating, and the color of the solution changed to dark yellow after 240 min. A similar change in color occurs after 240 min when using glycated chicken Mfs with maltose (Nishimura et al., 2015; Nishimura and Saeki, 2016). Although thermal gel-formation was observed using glycated chicken Mfs with maltose, ribose-conjugated chicken Mfs did not form a gel during 240 min of heating, despite having the same protein concentration (15 mg/mL).
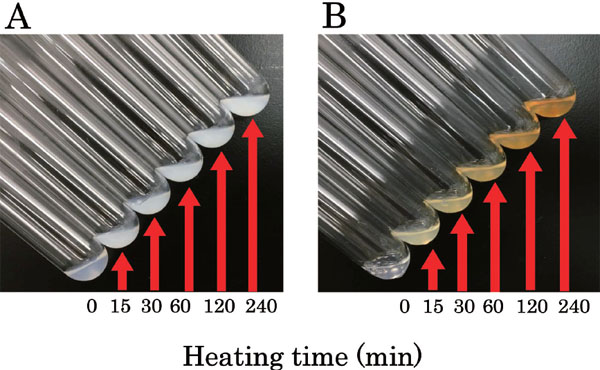
Effect of heating on both Mfs.
Chicken Mfs without or with ribose (a ribose mixing ratio of 10.7 for Mfs (w/w)) were incubated at 38°C and 40% RH for 4.55h, and then unmodified Mfs (A) and the optimized ribose-conjugated chicken Mfs (B) were prepared and dissolved in 0.5 M and 0.1 M NaCl solution (pH7.5), respectively. Both chicken Mfs (0.5 mL) containing 15 mg of protein/mL was placed in a test tube (diameter 16 mm) and heated at 90°C in water bath for 240 min. The change of each chicken Mfs was followed. The status of Mfs solutions was checked by tilting the test tubes at an angle.
In a previous study (Nishimura and Saeki, 2016), the thermal gel of maltose-conjugated chicken Mfs collapsed by subsequent heating at 90°C. We thought that this phenomenon might be due to the inhibition of thermal gel-formation due to the presence of some products of the Maillard reaction. Actually, in this study, rapid coloration over time was observed. The Maillard reaction may have advanced further in the optimized glycated chicken Mfs solution during heating due to the high reactivity of the five-member ribose sugar ring. Accordingly, more Maillard reaction products were quickly generated in our study when compared to previous studies (Nishimura et al., 2015: Nishimura and Saeki, 2016), resulting in inhibition of thermal gel-formation. This phenomenon suggests that optimized glycated chicken Mfs with ribose are not suitable for use in processed food requiring gel-formation of Mfs. However, success in the solubilization of Mfs could lead to development of drinks with high meat content, and thus greater nutritional value.
Changes in protein subunit composition of chicken Mfs with or without ribose during heating at 90°C Unmodified and optimized glycated chicken Mfs solutions were heated at 90°C and changes in the protein subunit composition were evaluated by SDS-PAGE (Fig. 4). In SDS-PAGE analysis of unmodified Mf in the absence of 2-ME (Fig. 4A), large molecular weight proteins stacked on the gel top before heating (0 h), indicating that a polymer component was formed during the mixing/lyophilization process. In addition, polymerization of protein subunits proceeded with heating, and MHC and actin gradually disappeared. In contrast, protein polymers were degraded in the presence of 2-ME (Fig. 4B) and protein subunits appeared, suggesting that disulfide (SS) bridges among the proteins were formed during heating. Similar changes in SDS-PAGE patterns were observed in previous studies (Nishimura et al., 2015; Nishimura and Saeki, 2016).

Changes in protein subunit of unmodified and optimized glycated chicken Mfs during heating.
Unmodified (A, B) and optimized glycated (C, D) Mfs were heated at 90°C for 240 min and then subjected to SDS-PAGE analysis in the absence (A, C) or the presence (B, D) of 10% 2-ME. MHC: myosin heavy chain.
In SDS-PAGE of the optimized glycated chicken Mfs before heating in the absence of 2-ME (Fig. 4C), glycated MHC, tropomyosin and actin were separated according to their molecular weights. Although the molecular weight of intact tropomyosin is lower than that of actin, there are greater amounts of lysine residues in tropomyosin than in actin (Nakamura et al., 2005); therefore, higher molecular weight proteins are brought to tropomyosin by binding to a greater number of ribose molecules (Fig. 4C, 0 min). These bands were almost lost after 60 min of heating. Moreover, the addition of 2-ME (Fig. 4D) did not have any marked influence on SDS-PAGE results, suggesting production of covalent cross-linkage among glycated Mf subunits.
Heat-induced gelation of myosin in high ionic strength medium can be represented by two reactions (Ishioroshi et al., 1979; Samejima et al., 1981; Numakura et al., 1985): i) aggregation of the globular head of the myosin molecule, which is complementary to and closely associated with the oxidation of the SH groups; and ii) the inevitable network formed by thermal unfolding of the helical tail. Such behavior was observed in maltose-conjugated chicken Mfs, which has thermal gel-forming ability. However, gel-formation was not observed after 60-min heating, despite the formation of cross-links observed by SDS-PAGE. We believe that increased products and intermediates of the Maillard reaction may have caused the collapse of the gel structure, as this reaction occurs during heating (Nishimura and Saeki, 2016). In this study, abundant generation of these products and intermediates and rapid interactions with MHCs would occur due to the high reactivity of ribose. Accordingly, thermal gel-formation would be inhibited and therefore not observed.
HORAC of the optimized glycated chicken Mfs Inhibition curves for the optimized glycated chicken Mfs are shown in Fig. 5. As the enrichment of high protein concentrations over 1.5 mg/mL was not reproducibly achieved, three regression lines obtained from each determination are shown. The residual ratio of •OH in native chicken Mfs in 2.0 mg of protein/mL was 85.6 ± 1.9% (n = 3), indicating that native chicken Mfs scarcely demonstrated HORAC, a phenomenon observed in a previous study (Nishimura and Saeki, 2016). In contrast, optimized glycated chicken Mfs demonstrated a reduced residual ratio of •OH with increasing protein concentration, giving three optimized glycated chicken Mfs IC50 values, with a mean of 1.3 ± 0.4 mg/mL (n = 3). HORAC of the optimized glycated chicken Mfs was converted into GA equivalent activity (µmol/mL) based on the GA IC50 value (6.4 ± 0.1 nmol/ mL), yielding a value of 5.1 ± 1.3 µmol of GA equivalent/g of protein (n = 3). Compared to the HORAC of the optimized glycated chicken Mfs with maltose (7.8 ± 1.0 µmol of GA equivalent/g of protein (n = 3)) (Nishimura and Saeki, 2016), the optimized glycated chicken Mfs with ribose obtained in this study produced a significantly low value (p < 0.05).
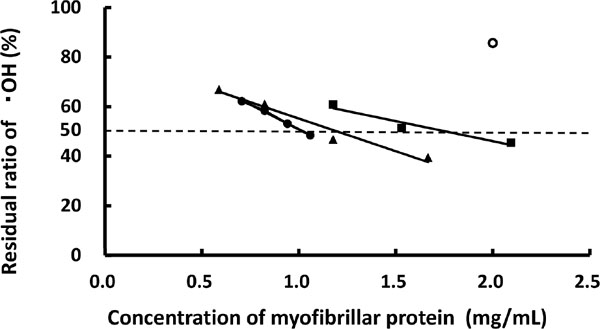
Antioxidant properties of glycated chicken Mfs
The antioxidant abilities of native chicken Mfs (○) and optimized glycated chicken Mfs with ribose against •OH were measured three time independently with the fluorescence method. The first (■), second (▴), and third (●) experiment preformed are presented separately. Values represent the mean ± standard deviation (n ≥ 3).
The optimized maltose-conjugated chicken Mfs formed a thermal gel after 30 min at 90°C, and produced a HORAC value of 4.4 ± 1.7 µmol of GA equivalent/g of protein (Nishimura and Saeki, 2016). Although the HORAC value of the optimized ribose-conjugated chicken Mfs in this study was lower than that of the maltose-conjugated chicken Mfs, the coloration advanced rapidly with heating (Fig. 3B). Heated samples provided a HORAC value of 113.0 ± 22.7 µmol of GA equivalent/g of protein (n = 3) (data not shown) after a 30 min-heating, producing approximately 25 times the thermal gel as maltose-conjugation chicken Mfs. This result may be attributed to the fast progression of the Maillard reaction because the solution state was maintained during heating due to the failure of gel-formation. This antioxidant ability was similar to that found in strawberries (Boxin et al., 2002).
The optimized ribose-conjugated chicken Mfs were soluble in a low ion strength medium but failed to form a thermal gel. However, they acquired a higher antioxidant ability when compared with the maltose-conjugated chicken Mfs when heated, suggesting that they could be used as an ingredient in nutritional supplement drinks derived from animal protein.
Acknowledgements The authors are grateful to assistant professor Ms. Yuki Nishimura, to master's course student Ms. Momoka Suzuki, and to the students of Doshisha Women's College of Liberal Arts for their assistance.
gallic acid
HORAChydroxyl radical antioxidant capacity
IC50half-maximal inhibitory concentration
2-ME2-mercaptoethanol
Mfsmyofibrillar proteins
MHCmyosin heavy chain
•OHhydroxyl radical
PAGEpoly-acrylamide gel electrophoresis
RCOrandom-centroid optimization
RHrelative humidity
RLUrelative light unit
SDSsodium dodecyl sulfate
SODsuperoxide dismutase
SOSAsuperoxide anion radical scavenging activity
SSdisulfide
Uunit