2018 Volume 24 Issue 6 Pages 1101-1109
2018 Volume 24 Issue 6 Pages 1101-1109
Endoplasmic reticulum (ER) stress and the resulting neuronal cell damage have been implicated in the development and progression of Alzheimer's disease. Tunicamycin (TM) induces ER stress in vitro, and the protective effects of the carotenoid zeaxanthin against the effects of TM have recently been studied. Zeaxanthin has been shown to hold several beneficial health properties in human studies. The protective role of zeaxanthin against the toxic effects of TM has recently been studied for the first time. The present study investigated whether zeaxanthin protects SH-SY5Y cells against TM-induced cell damage in vitro. Both pretreatment and post-treatment with zeaxanthin increased cell viability and suppressed lactate dehydrogenase release compared to levels in controls. Further, we found that zeaxanthin considerably ameliorated TM-induced cell damage by protecting the integrity of the mitochondrial membrane decreasing caspase-3 activity, and by reducing the apoptosis rate as well as the expression of the ER stress biomarker GRP78. Modulation of GRP78 may be one of the mechanisms by which zeaxanthin affects cell viability. Our results indicate a potential application of zeaxanthin in the development of new therapeutic drugs for the treatment of Alzheimer's disease.
Alzheimer's disease (AD) is the most common cause of dementia, affecting approximately 45 million people in the world (Cao et al., 2018; Gauthier et al., 2018), but its underlying mechanism remains largely unknown. Recently, several studies have shown that dysfunction of the endoplasmic reticulum (ER) triggers endoplasmic reticulum stress (ER stress) and leads to many protein-folding diseases, including AD (Gerakis and Hetz, 2018; Scheper and Hoozemans, 2015).
Short-term ER stress is an adaptive mechanism that attenuates ER tension by adjusting the unfolded proteins in the ER (Doyle et al., 2011; Hetz and Saxena, 2017). When ER stress is triggered, unfolded protein signaling pathways are activated to rebuild of ER homeostasis (Bravo et al., 2013). However, long-term ER stress complicates the rebuilding of ER homeostasis and leads to cell death (Nishitoh, 2012; Salminen, Kauppinen, Suuronen, Kaarniranta, and Ojala, 2009). The loss of neurons in the brain is a risk factor promoting the formation and development of AD pathology. Therefore, regulation of ER stress is regarded as a new strategy for AD treatment (Kehoe, 2018). Nutraceutical molecules that ameliorate ER stress may serve as potential drugs to prevent disease development.
Several studies have reported that compounds present in food possess neuroprotective properties (Ghosh et al., 2015; Murillo et al., 2010). Recently, the relationship between the large class of plant pigments known as carotenoids and cognitive health throughout life has been a focal point of research, because carotenoids are selectively taken up into brain tissue (Fiedor and Burda, 2014; Obulesu et al., 2011). Zeaxanthin is a carotenoid that could mitigate human diseases, such as cancer, macular degeneration, and angiocardiopathy (Johnson, 2014). Several epidemiologic studies have shown that higher levels of zeaxanthin in the diet are associated with lower risks of cognitive dysfunction. A recent study indicated that the β-rings present in the chemical structure of zeaxanthin inhibit the amyloid beta aggregation characteristic of AD in a dose-dependent manner, which suggests the great potential of zeaxanthin as a neuroprotective agent in AD treatment (Lakey-Beitia et al., 2017). Accumulating evidence indicates that tunicamycin (TM) can be used to induce ER stress in vitro (Xing et al., 2016). Moreover, human neuroblastoma SH-SY5Y cells are widely used to establish AD cell models for research releted to neurological diseases (Yang et al., 2016).
Therefore, the aim of this research was to investigate the neuroprotective effect of zeaxanthin against TM-induced cytotoxicity in SH-SY5Y cells, which could potentially provide a new direction for the application of zeaxanthin in the treatment and prevention of AD.
Cell culture and drug treatment The human SH-SY5Y cells used in this study were purchased from the Chinese Academy of Medical Sciences (Beijing, China). SH-SY5Y cells were cultured in RPMI 1640 medium (Invitrogen, Gibco, CA, USA) as described previously (Xin et al., 2017). Cells were added into culture dishes and allowed to reach at least 80% confluence before further treatment, then divided into three groups: a normal group (control), a TM group treated with tunicamycin (Huayueyang Biotechnology, Beijing, China) at 0.25–10 µg/mL concentrations, and a zeaxanthin treatment group treated with zeaxanthin (Shanghai Yuan Ye Biotechnology, Shanghai, China) dissolved in ethanol and diluted in RPMI 1640 at 2–100 µM concentrations. Cells were incubated with zeaxanthin for different times periods before exposure to TM.
3(4,5dimethylthiazol2yl)2,5diphenyltetrazolium bromide (MTT) assay Briefly, SH-SY5Y cells were seeded into 96-well plates at a density of 1×105 cells/well, and incubated overnight. Next, cells were treated with drugs for an appropriate amount of time. Cell viability was measured using an MTT assay kit (Sigma-Aldrich, St. Louis, MO, USA) as previously described (Xu et al., 2016).
Lactate dehydrogenase (LDH) assay The amount of lactate dehydrogenase (LDH) released into the culture medium was used to measure cell cytotoxicity. After experimental treatment, Cytotoxicity was measured using an LDH assay kit (Jiancheng Institute, Nanjing, China). Control cells were exposed to 3% TritonX-100 (let all LDH release into medium) and LDH activity was assessed (defined as 100%). LDH activity in the other groups was calculated as a percentage of that in the control group.
Measurement of mitochondrial transmembrane potential (µm) and caspase-3 activity JC-1 dye (Solarbio) was used to measure changes in µm. In brief, after experimental treatment, the intensity of red fluorescence and green fluorescence were measured. µm was expressed as the ratio of JC-1 red/green fluorescence intensity, and the value was normalized to that of the control group. In addition, the images were captured under a fluorescent microscope. In the same way, caspase-3 activity was measured using the commercially available Caspase-3 Assay kit (Beyotime Biotech, Shanghai, China). Caspase-3 activity in the TM group was defined as 100%. Caspase-3 activities were normalized to that of the TM group.
Flow cytometry Following experimental treatment, cell apoptosis was measured using an Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide Apoptosis Detection Kit (Gen-View, El Monte, CA, USA) as described previously (Olivera Santa-Catalina et al., 2017). Samples were analyzed by flow cytometry (BD Biosciences, Franklin Lakes, NJ, USA).
Western blotting The protein was extracted from cultured SH-SY5Y cells using ice-cold RIPA radio immuno precipitation assay buffer lysisbuffer (containing 0.1% PMSF) (Beyotime Biotech). Protein concentration was measured using a Bradford protein assay kit (Dingguo Changsheng Biotech Co., Ltd., Beijing, China). Western blotting was performed as previously described (Ahmadi et al., 2017). Approximately 30–50 µg of protein was separated on 8% polyacrylamide gel, then transferred to polyvinylidene difluorid membranes and incubated with antibodies against GRP78 (Cell Signaling Technology, Inc., Massachusetts, USA) and β-actin (Sigma-Aldrich). The immunoblots were visualized using a calibrated densitometer (Bio-Rad, Hercules, CA, USA). The intensity of the bands was quantified using the Quantity One software (Bio-Rad).
Statistical analysis GraphPad Prism 6.01 (GraphPad Software, Inc., La Jolla, CA, USA) was used, and all data were analyzed using SPSS 20.0 (SPSS, Inc., Chicago, IL, USA). Values are expressed as mean ± standard deviation, and each experiment was performed at least three times. Statistical analysis was performed by one-way ANOVA. Differences were compared using leastsignificant different (LSD) post-hoctests. P < 0.05 was considered statistically significant.
Zeaxanthin inhibited TM cytotoxicity toward SH-SY5Y cells in a concentration-dependent manner First, we explored the protective effect of zeaxanthin against cell damage induced by TM, and cell viability was examined by the MTT assay. As shown in Figure 1B, TM treatment at concentrations over 2 µg/ mL significantly reduced cell viability compared to that of control cells (p < 0.01). At TM concentrations of 4 and 6 µg/ mL, SH-SY5Y cell viability percentages were 68.97% and 47.73%, respectively; thus, 5 µg/mL TM was chosen for the following experiment due to the 30–40% observed cell death.
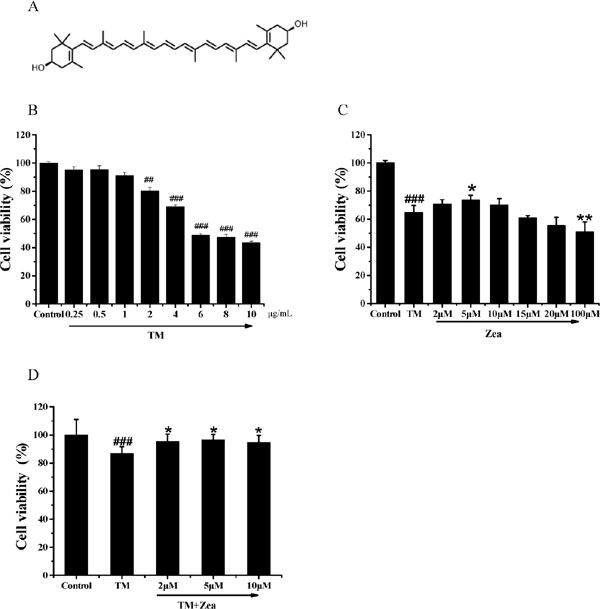
Effect of zeaxanthin on SH-SY5Y cell viability damaged by TM. (A) The structure of zeaxanthin. (B) Cells viability of SH-SY5Y cells after treated with TM (0.25–10 µg/mL) for 36 h (N=3). (C) Cells survival of SH-SY5Y cells was measured after pre-treat with zeaxanthin (2–10 µM) for 6 h and then incubated with 5 µg/mL TM for a futher 36 h (N=3). (D) Cells survival of SH-SY5Y cells was measured after 30 min exposure to 5 µg/mL TM and then post-treated with zeaxanthin (2, 5,10 µM) for a futher 24 h (N=3). ##P < 0.01, ###P < 0.005 versus control group; *P < 0.05, **P < 0.01 versus TM group.
We further investigated cell viability changes in cells treated with zeaxanthin. SH-SY5Y cells were cultured with zeaxanthin for 6 h before exposure to TM for 36 h. As shown in Figure 1C, pretreatment with zeaxanthin attenuated TM-induced cell death, and 5 µM zeaxanthin was the most effective concentration. Next, we investigated whether post-treatment with zeaxanthin could suppress cell death induced by TM. Figure 1D shows that post-treatment with zeaxanthin rescued the cells from TM-induced injury. The protective effect of zeaxanthin was further verified by the LDH assay. Compared with the TM group, cells pre-treated with 5 µM zeaxanthin for 6 h exhibited significantly less TM-induced LDH leakage (Figure 2A), and similar results were obtained after post-treatment with zeaxanthin (Figure 2B).
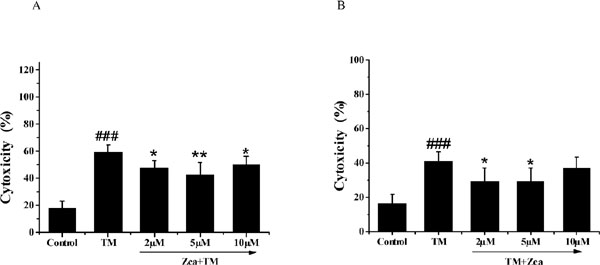
Effect of zeaxanthin on LDH activity of SH-SY5Y cells damaged by TM. (A) Cells cytoxicity of SH-SY5Y cells was measured after pre-treated with zeaxanthin (2 µM, 5 µM, 10 µM) for 6 h and then incubated with 5 µg/mL TM for a futher 36 h (N=3). (B) Cells cytoxicity of SH-SY5Y cells was measured after incubated with 5 µg/mL TM for 30 min and post-treated with zeaxanthin (2 µM, 5 µM, 10 µM) for 24 h (N=3). ###P < 0.005 versus control group; *P < 0.05, **P < 0.01 versus TM group.
Zeaxanthin ameliorated TM cytotoxicity toward SH-SY5Y cells in a time-dependent manner We then evaluate the effect of zeaxanthin treatment time on cell survival. SH-SY5Y cells were cultured with zeaxanthin (5 µM) for 1–24 h, followed by treatment with TM for 36 h before cell viability was measured. As shown in Figure 3A–D, pretreatment with zeaxanthin for 1–3 h had no significant effects, whereas the protective effect was significant at pretreatment times of 4–24 h, and especially at 6 h. Thus, the optimum pretreatment time of 6 h was used in the following experiment.
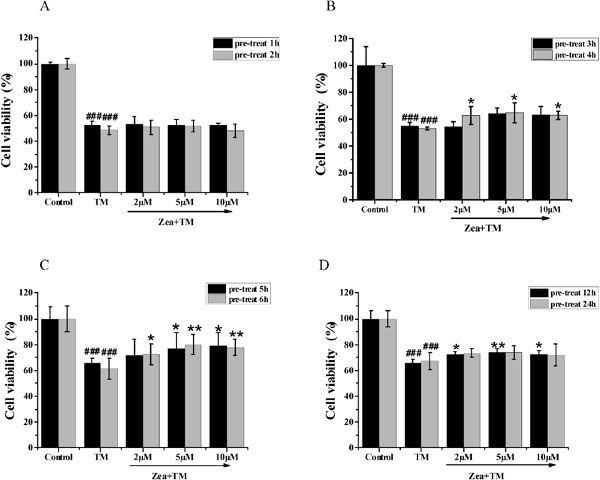
Effect of pre-treat time of zeaxanthin on cell viability of SH-SY5Y cells damaged by TM. Zeaxanthin (2 µM, 5 µM, 10 µM) was added 1 h or 2 h (A), 3 h or 4 h (B), 5 h or 6 h (C), and 12 h or 24 h (D) prior to treatment of the cells with TM (5 µg/mL). Cell viability were measured by MTT assay (N=3). ###P < 0.005 versus control group; *P < 0.05, **P < 0.01 versus TM group.
Zeaxanthin restored changes in mitochondrial membrane potential (µm) induced by TM We next assessed µm alteration by JC-1 staining. JC-1 staining analysis showed that µm significantly increased (from 62.8% to 83.5%) under zeaxanthin treatment (5 µM) compared to that in the TM group (Figure 4B). Additionally, we found that zeaxanthin significantly reduced activation of the apoptosis marker caspase-3 by 58% compared to that in the TM group (Figure 4C).
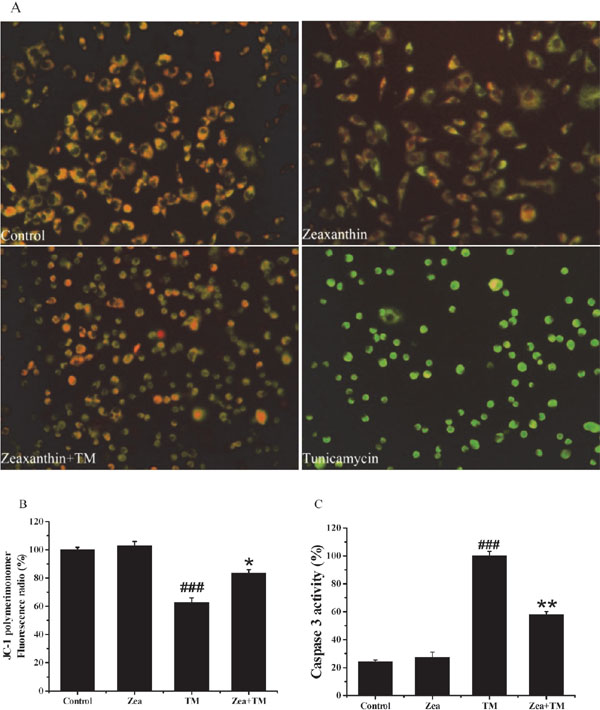
Effect of zeaxanthin on mitochondrial membrane potential (Δψm) and caspase 3 activity of SH-SY5Y cells damaged by TM. SH-SY5Y cells were pre-treat with zeaxanthin (5 µM) for 6 h , and then cultured with or without 5 µg/mL TM for 36 h. After that, JC-1 assay and caspase 3 activity assay were performed (N=3). (A) Images were captured under a fluorescence microscope. (B) Histograms of the percentages of the red/green fluorencent intensity radio of JC-1 staining. (C) Histograms of the caspase 3 activity. ###P < 0.005 versus control group; *P < 0.05, **P < 0.01 versus TM group
Zeaxanthin decreases TM-induced apoptosis of SH-SY5Y cells Accumulating evidence has revealed that ER stress inhibits cell growth and induces apoptosis. To investigate whether zeaxanthin suppresses TM-induced apoptosis, the apoptosis rate was measured by the annexin V-FITC/PI detection kit and flow cytometry. As shown in Figure 5, treatment with 5 µM zeaxanthin significantly decreased the apoptotic (early apoptotic, late apoptotic, total apoptotic) rate compared to that in the TM group. These results indicated that zeaxanthin (5 µM) attenuated the TM-induced apoptosis of SH-SY5Y cells.
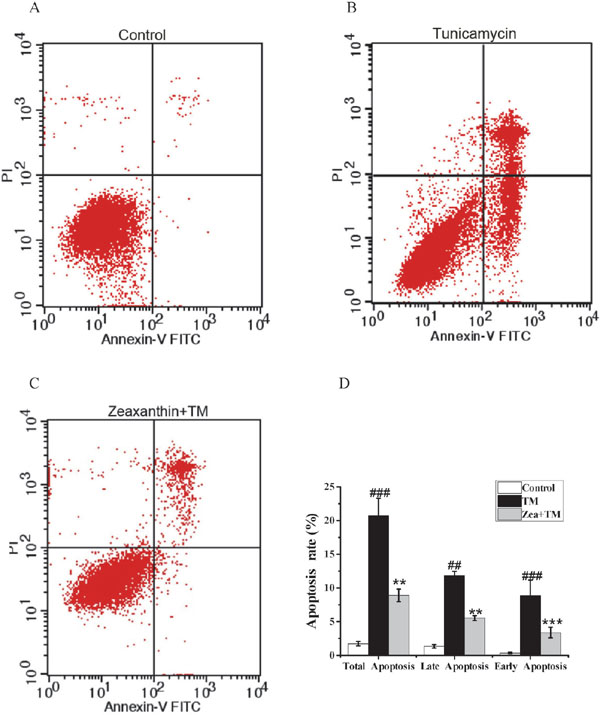
Effect of zeaxanthin on the apoptosis rate of SH-SY5Y cells damaged by TM. Zeaxanthin (5 µM) were added 6 h before exposing the cells to the TM (5 µg/mL). (A) Dot plot of cells in the control group. (B) Dot plot of cells in the TM group. (C) Dot plot of cells in the zeaxanthin group. (D) Apoptosis rate (early apoptotic, late apoptotic, total apoptotic) of each group (N=3). ##P < 0.01, ###P < 0.005 versus control group; **P < 0.01, ***P < 0.005 versus TM group.
Effect of zeaxanthin on GRP78 expression in SH-SY5Y cells Additionally, previous studies have indicated that GRP78 expression is upregulated in AD (Penke et al., 2016). We therefore investigated whether GRP78 is involved in the zeaxanthin-induced neuroprotective effects on SH-SY5Y cells. Western blot analysis revealed that zeaxanthin (5 µM) significantly reduced GRP78 protein levels compared with those in the TM group (Figure 6).
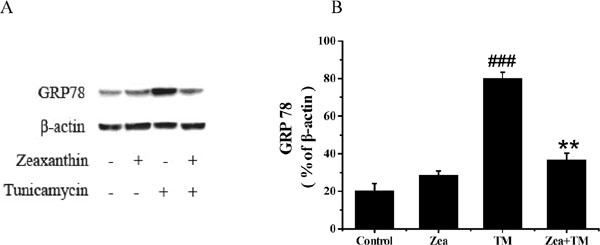
Expression of GRP78 in SH-SY5Y cells after TM with or without zeaxanthin treatment. (A) GRP78 and β-action expression was detected by Western blot. (B) Histograms of the relative amount of GRP 78 based on the Western blotting (N=3). ###P < 0.005 versus control group; **P < 0.01 versus TM group.
In this work, we investigated the neuroprotective effect of zeaxanthin against TM-induced death in SH-SY5Y cells. Moreover, our study is the first to demonstrate that certain concentrations of zeaxanthin protect and rescue neuronal cells from damage induced by TM (an ER stress inducer). Further experiments showed that zeaxanthin reduced apoptosis and alleviated ER stress.
With the aging of the population, the rapid increase in incidence of AD has become an important issue in society (Shah et al., 2016); however, there has been no fundamental breakthrough in drug development or clinical treatment of AD. Recent research has focused on prevention in the early stages rather than later treatment of AD (Suominen et al., 2015). Additionally, ER stress has been recently shown to occur during the early stages of AD (Hashimoto et al., 2018). Numerous experiments have reported that ER stress induces apoptosis both in vivo and in vitro (Liu et al., 2018; Xiao et al., 2016); therefore, inhibition of ER stress-induced apoptosis has become a focal point of research.
A number of studies have indicated that some carotenoids have neuroprotective effects on nerve cells (Papandreou et al., 2006). Zeaxanthin is an isoprenoid carotenoid that can pass through the blood-brain barrier to be taken up into neural tissue (Johnson, 2014), and it has been shown to possess anti-amyloid properties (Lakey-Beitia et al., 2017). Additionally, the accumulation of amyloid beta is closely associated with the formation of AD (Lu et al., 2013). Besides, previous study have shown that both lutein and zeaxanthin suppress ER stress in ARPE-19 cells exposed to a high glucose environment (Ying et al., 2017). Moreover, an in vivo study revealed that diabetes-induced cognitive impairment can be improved with zeaxanthin treatment in rats (Zhou et al., 2017).
Based on the above discoveries, we explored whether zeaxanthin treatment could attenuate SH-SY5Y cell death due to ER stress. Our results revealed that the survival rate of cells decreased in a concentration-dependent manner with TM treatment for 36 h. When pretreated with zeaxanthin, the cell survival rate increased (Figure 1), and further experiments suggested that the neuroprotective effect of zeaxanthin was related to culture duration (Figure 3). Additionally, our results showed that the decrease in cell viability and increase in LDH release induced by TM were significantly inhibited by zeaxanthin, indicating that zeaxanthin provides protective effects on SH-SY5Y cells (Figure 2).
The mitochondria are an important apparatus associated with several neurological diseases (Leitao-Rocha et al., 2015; Pareyson et al., 2015). Increased mitochondrial DNA damage in neurons has been found in AD patients, indicating that mitochondrial damage may be critically involved in the progression of AD (Lv et al., 2014). In this study, we found that TM-induced µm loss was restored by zeaxanthin. Additionally, pretreatment with zeaxanthin reduced the caspase-3 activation (Figure 4). These results suggest that zeaxanthin protects SH-SY5Y cells by decreasing the apoptosis rate and restoring mitochondrial morphology.
Neuronal death is a pathological hallmark of AD. There are several forms of neuronal death: necrosis, apoptosis, and pyroptosis; among them, apoptosis is believed to be the most common cell death pathway in AD (Obulesu and Lakshmi, 2014). In this work, we found that zeaxanthin (5 µM) significantly reduced apoptosis induced by TM. These datas suggest that the protective effect of zeaxanthin on SH-SY5Y cells is related to the apoptosis pathway.
Protein kinase RNA-like ER kinase (PERK), inositol-requiring protein 1 α (IRE1α), and activating transcription factor 6 (ATF6) are three major ER stress sensors that interact with GRP78 under normal conditions. However, when ER stress is triggered, GRP78 is detached from the three sensors and subsequently interacts with abnormal proteins to facilitate their refolding and secretion from the ER (Park et al., 2017). Our results showed that GRP78 significantly decreased after addition of zeaxanthin (5 µM) compared with levels in the TM group (Figure 6). Therefore, we concluded that zeaxanthin may provide its protective effect against TM-induced apoptosis by blocking the dissociation of GRP78 and the ER stress sensors.
The most striking finding of this research was that zeaxanthin functions as a modulator of the cell survival signaling pathway and reduces TM-induced damage to SH-SY5Y cells through the regulation of LDH release, µm, and caspase-3 activation, there by decreasing cell apoptosis. To our knowledge, this is the first report that zeaxanthin suppresses TM-induced apoptosis in SH-SY5Y cells by blocking the dissociation of GRP78 from ER stress sensors; however, mechanistic studies and animal experiments will be required to confirm these findings.
In this study, we provided evidence for a possible mechanism by which zeaxanthin protects against cell death. Our results suggest that zeaxanthin ameliorates TM-induced cell damage by blocking the dissociation of GRP78 from ER stress sensors. These data encourage further research on the ATF6, IRE1β, and PERK signaling pathways, particularly regarding their association with GRP78. Such studies shed light on the therapeutic potential of zeaxanthin in combating neurodegenerative diseases, including AD.
The authors have no conflict of interest to anybody or institution.
Acknowledgement We are grateful to Professor Yinghui Shang and Hanchang Huang and of Beijing Union University for excellent technical assistance. This study was supported by National Natural Science Foundation of China (31471587).