2019 Volume 25 Issue 1 Pages 75-79
2019 Volume 25 Issue 1 Pages 75-79
Heating vegetable oil in a microwave oven can lead to the formation of reactive free radicals, which are responsible for causing lipid oxidation. The thermo-oxidative stability of olive oil has previously been studied under microwave heating for short processing periods that simulate typical cooking times. This study compares the effect of the oxidative degradation of extra virgin olive oil (EVOO) during extended heating times under different microwave powers (150 and 500 W) or conventional heating. The results showed that intense microwave heating of EVOO significantly increased the free acidity and specific extinction coefficients (K270), and resulted in more severe loss of phenolic compounds, compared with conventional heating. Microwave treatment also resulted in higher amounts of acrolein in EVOO after a short processing period compared with conventional heating. These results demonstrate that intene microwave heating has a more severe effect on EVOO compared with conventional heating.
Modern food processing technologies have resulted in procedural changes to cooking. The microwave oven is a common kitchen appliance that uses electromagnetic radiation for a broad range of food processing applications, such as heating, drying, baking, sterilizing, and defrosting food, and continues to become simpler and more convenient. Microwave heating has substantial advantages over conventional heating, especially in terms of energy efficiency. Domestic microwaves are often used at powers of 150, 500, and 700 W, selected according to the required food processing technique.
Microwave heating of different vegetable oils and fats causes the formation of free radicals that rapidly react with atmospheric oxygen to produce hydroperoxides and secondary oxidation products (Albi et al., 1997b; Farag et al., 1992; Hassanein et al., 2003). The oxidative degradation of oils caused by microwave heating can reduce their sensory and physicochemical quality. For example, acrolein (2-propenal), which has a well-known undesirable and irritating odor, can be formed in heated oils and autoxidized lipids (Faroon et al., 2008; Gasee et al., 1996; Li and Holian, 1998; Uchida, 1999).
Olive oil is core to the Mediterranean diet. More recently, olive oil has been used in many Asian countries as a premium edible oil owing to its beneficial health effects (Capogna and Gomez, 2016). In particular, extra virgin olive oil (EVOO), which is produced by mechanically pressing olive fruit without using chemicals or solvents, is the highest grade of olive oil. EVOO has a free acidity (FA), expressed as the oleic acid content, of not more than 0.8 g/100 g oil, while other characteristics correspond to those established for this category in the International Olive Council (IOC) standards (IOC, 2018). EVOO has a high biological value due to its monounsaturated oleic acid content, which is considered a healthy fat, and its antioxidant content. The main antioxidants in EVOO are phenolic compounds, the quantity of which is important for evaluating olive oil quality. Olive oil polyphenols also play a major role in preventing certain chronic human diseases (Visioli and Galli, 2002).
Previous studies have described the effect of microwave heating on the thermo-oxidative stability of olive oil during short periods (up to 15 min) that simulated typical cooking times (Caponio et al., 2002; Cerretani et al., 2009; Malheiro et al., 2009). In general, olive oil is used for cooking meat, poultry, seafood, and large cuts of vegetables. Dense foods such as these should be cooked on medium power (500 W) for longer periods. This allows heat to reach the center without overcooking outer areas. However, little attention has been given to the effect of extensive microwave heating times and different microwave powers on the oxidative degradation of olive oil. The present study aims to compare differences in physicochemical parameters and acrolein formation as a sensory characteristic due to prolonged microwave and conventional heating of EVOO. The overall objective is to determine the unique effects of microwave heating on the deterioration of EVOO, as compared with conventional heating. The effects of microwave and conventional heating methods on the quality attributes of EVOO are compared by adjusting the heating conditions, such as the temperature, highest attained temperature, and EVOO content.
Materials EVOO from cultivar Nevadillo blanco was obtained by dual-phase decanter centrifugation using industrial processors (Kishimoto and Kashiwagi, 2018). Medium-chain triglyceride (MCT) oil (The Nisshin OilliO Group, Ltd., Tokyo, Japan) was purchased at a market. Acrolein (purity, >95 %) was purchased from Tokyo Kasei Industry (Tokyo, Japan).
Heating procedures Three samples (50 g) for each treatment were weighed into petri dishes (diameter: 10 cm, height: 2 cm). The microwave oven (Panasonic NE-EH229, Osaka, Japan) was operated at 500 or 150 W. Samples were heated in the microwave oven at the center of the rotating plate (diameter, 28 cm) for 2.5, 5, 10, 15, 20, 25, and 30 min, with three independent experiments performed under the same conditions. For conventional heating, three samples (200 g) were weighed into a frying pan (diameter: 20 cm, height: 4.5 cm). These samples were heated in a conventional oven with the temperature regulated to a maximum of 200 °C. All samples were cooled rapidly and stored in sealed tubes at 17 °C until analysis. After each heating period, the oil temperature was determined with a Testo 270 Deep-Frying Oil Tester (Testo AG, Lenzkirch, Germany).
Analytical procedures FA, total phenolic content, and the specific extinction coefficient (K270) for the oil samples were measured using an OxiTester (CDR, Ginestra Fiorentina, Italy) (Kamvissis et al., 2008). FA was determined using the OxiTester method, which had been preliminarily confirmed against the official method of analysis using oil samples over a wide range of values (Gucci et al., 2012). Aliquots of the oil sample (2.5 µL for measuring FA and 10 µL for measuring total phenolic content and K270) were added to a prefilled cuvette.
Flash gas chromatography electronic nose analysis The headspace of the oil samples was analyzed using a HERACLES Ⅱ electronic nose (Alpha MOS, Toulouse, France) (Kishimoto et al., 2017) equipped with two identical gas chromatography columns working in parallel mode, namely a non-polar column (MXT-5; length, 10 m; diameter, 180 µm) and a polar column (MXT-WAX; length, 10 m; diameter, 180 µm), that produced two chromatograms simultaneously. The electronic nose was also equipped with an HS100 autosampler (CTC Analytics AG, Zwingen, Switzerland) to automate sample incubation and injection. For calibration, an alkane mixture (from n-heptane to n-hexadecane) was used to convert retention times into Kovats indices. The analytical conditions were as follows: an aliquot of oil sample (2.0 g) was placed in a 20-mL vial, sealed with a magnetic cap, and placed in the autosampler, which was then placed in a HERACLES shaker oven and incubated for 15 min at 60 °C with shaking at 500 rpm. The headspace was sampled (5 mL) using a syringe and then injected into the gas chromatograph. The thermal program started at 40 °C (held for 10 s) and then increased to 250 °C at a rate of 1.5 °C/s. The final temperature was held for 60 s and the total separation time was 120 s. The data were acquired and processed using AlphaSoft v14.5 software (Alpha MOS, Toulouse, France). The AroChemBase module (Alpha MOS, Toulouse, France) was used to identify the volatile compounds.
Quantification of acrolein To determine the amount of acrolein in the oil samples, a standard curve was first established (Kishimoto and Kashiwagi, 2018). Different concentrations of acrolein were prepared in MCT oil then subjected to flash gas chromatography electronic nose analysis. Amounts of acrolein in EVOO samples after microwave heating were determined from the standard curve.
Statistical analyses The data are presented as mean ± standard deviation from three replicates and analyzed by one-way analysis of variance (ANOVA) followed by the Tukey–Kramer test in Microsoft Excel. Values of p < 0.05 were considered statistically significant.
The temperature increase during microwave and conventional heating of EVOO is shown in Fig. 1. The temperature increased with increasing heating time for both heating methods. Conventional heating showed a similar increase to that for intense microwave heating at 500 W, reaching 207 °C. For weak microwave heating at 150 W, the temperature reached 124 °C.

Change in temperature of EVOO during microwave and conventional heating. Data are means ± SD (n = 3). a,b Mean values with different letters for heating for 30 min are significantly different (p < 0.05, Tukey–Kramer multiple comparisons test).
The FA value results from fatty acid hydrolysis. Figure 2 shows changes in FA during microwave and conventional heating. For conventional and weak microwave heating of EVOO, no increase in the FA value was observed during treatment. No significant differences among the FA values for EVOO subjected to intense microwave and conventional heating were observed up to 10 min, which simulated typical cooking times. The obtained results were consistent with previous reports (Cerretani et al., 2009; Malheiro et al., 2009). In contrast, the FA value significantly increased for prolonged intense microwave heating, which was initially less than 0.8 % for EVOO (IOC, 2018). These observations suggested that intense microwave heating can induce a higher level of EVOO degradation than conventional heating.
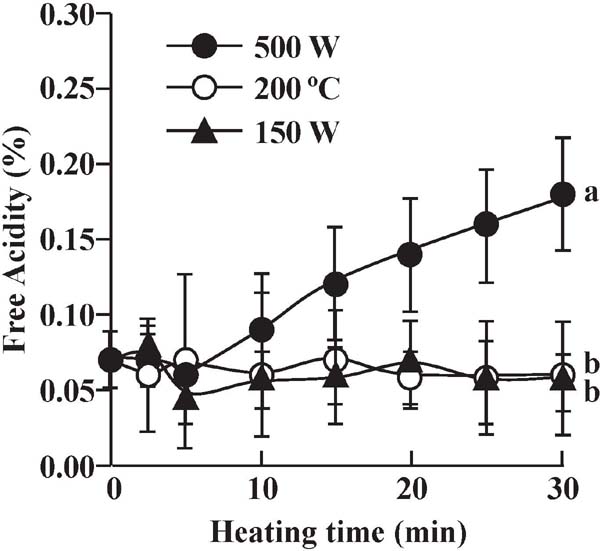
Change in free acidity of EVOO during microwave and conventional heating. Data are means ± SD (n = 3). a,b Mean values with different letters for heating for 30 min are significantly different (p < 0.05, Tukey–Kramer multiple comparisons test).
The peroxide value is a widely used indicator of fat oxidation, measuring lipid peroxides and hydroperoxides formed during the initial stages of oxidation. However, the peroxide value is not a good index for measuring oxidation because hydroperoxides are unstable when heated at high temperatures. The degree of olive oil oxidation can be expressed in terms of the specific extinction coefficient at 270 nm (K270), which is indicative of the presence of carbonyl compounds (Malheiro et al., 2009). The maximum K270 value permitted for EVOO is 0.22 (IOC, 2018). Figure 3 shows changes in K270 during microwave and conventional heating. Before the analysis, the EVOO-tested K270 value was 0.133. Conventional and intense microwave heating increased the K270 values with increasing heating time, in a manner similar to that for temperature (Fig. 1). In the initial heating stages (approximately the first 2.5 min), the K270 value showed little variation, which is in accordance with previous observations (Albi et al., 1997a; Caponio et al., 2003; Malheiro et al., 2009). The K270 values were more than 0.22 for EVOO after intense microwave and conventional heating for 10 min, indicating accelerated oxidation of EVOO. In the late heating stages (approximately the last 20 min), the values for intense microwave heating were slightly higher than those for conventional heating. Furthermore, weak microwave heating produced little change during the treatment, giving a K270 value of less than 0.22 for EVOO. These observations suggest that the degree of EVOO oxidation might depend on the heating temperature.

Change in K270 value of EVOO during microwave and conventional heating. Data are means ± SD (n = 3). a–c Mean values with different letters for heating for 30 min are significantly different (p < 0.05, Tukey–Kramer multiple comparisons test).
Figure 4 shows changes in the phenolic content during microwave and conventional heating. The phenolic content in EVOO decreased with increasing heating time under both microwave and conventional heating. A small reduction in phenolic content was observed after intense microwave heating of EVOO for a short processing period, as was also reported by Brenes et al. (2002) and Cerretani et al. (2009). Intense microwave heating of EVOO for 10 min resulted in a more severe loss of phenolic content (50 %) compared to conventional heating (25 %), after which the phenolic content decreased rapidly. After heating for 30 min, the losses for intense microwave and conventional heating were 88 % and 61 %, respectively. Weak microwave heating of EVOO for 30 min caused only minor losses in phenolic content (30 %). These observations suggest that the effect of intense microwave heating was distinct from that of conventional heating, and that degradation of phenolic compounds during prolonged and intense microwave cooking was not beneficial for the intake of olive polyphenols, such as the nutritional components in EVOO. These observations indicate that intense microwave heating can accelerate the degradation of higher levels of phenolic compounds in EVOO.

Change in phenolic contents of EVOO during microwave and conventional heating. Data are means ± SD (n = 3). a–c Mean values with different letters for heating for 30 min are significantly different (p < 0.05, Tukey–Kramer multiple comparisons test).
Owing to the strong impact of acrolein on flavor deterioration, the formation of this key volatile compound during heating should be monitored in order to evaluate the sensory quality of lipids susceptible to oxidative degradation. Figure 5A shows a representative chromatogram of the headspace gases obtained from MCT containing acrolein and EVOO after intense microwave heating for 10 min using the MXT-WAX column. The eluted acrolein peak occurred at a retention time of 25 s (Kishimoto and Kashiwagi, 2018). Peaks for the major volatile compounds of microwaved EVOO were also detected at the same retention time. Figure 5B shows changes in the acrolein content during microwave and conventional heating. EVOO can form small amounts of acrolein after heating at 180 °C (Fulluana et al., 2004; Kartragadda et al., 2010; Molina-Garcia et al., 2017; Kishimoto and Kashiwagi, 2018) because linolenic acid is usually present at a level of less than 1 % (IOC, 2018; Endo et al., 2013). The amount of acrolein in the conventionally heated EVOO gradually increased with increasing heating time, reaching 2,300 ppb after heating for 30 min. This indicated that heating at a higher temperature (>200 °C) induced acrolein formation, even from EVOO. The amount of acrolein in intensely microwaved EVOO quickly reached the maximum (2,500 ppb) after heating for 10 min, after which the acrolein level decreased rapidly. The triglycerides hydrolyze into glycerol and free fatty acids during heating with vegetable oils. Acrolein may be formed by the dehydration of the glycerol (Fritsch, 1981). The dehydration rate under microwave activation is much higher than those for conventional thermal activation (Calvino-Casilda et al., 2010). Acrolein is a very reactive monomer with a high tendency to polymerize. Excess formation of acrolein is thought to increase the polymerization rate. Rapid decreases in acrolein in intensely microwaved EVOO may be due to microwave-induced polymerization of acrolein. Conversely, weak microwave treatment produced only a very small increase in acrolein formation (28 ppb) after heating for 30 min. These observations indicate that intense microwave heating deteriorated the sensory quality of EVOO after even short processing periods. This might be due to a combination of temperature and energy effects induced by microwave treatment.

(A) A representative chromatogram of headspace gases obtained from MCT containing acrolein and EVOO after intense microwave heating for 10 min and (B) the formation of acrolein from EVOO during heating. The results of acrolein formation with different treatments were compared at the same heating time. Data are means ± SD (n = 3). Data marked with different letters represent significant differences.
Based on this comparative study, intense microwave heating at 500 W produced a significantly greater increase in FA and K270, large losses in phenolic compounds over a long treatment time, and higher amounts of acrolein in EVOO over a short processing period than those produced by lower-power microwave treatment (150 W) and traditional heating. These results confirm the deteriorative effect of prolonged and intense microwave heating, which can induce the oxidative degradation of EVOO. These findings may help to select the microwave power and exposure times that increase the intake of olive polyphenols and maintain the quality of EVOO for domestic cooking.
Acknowledgements We thank Simon Partridge, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.