2019 Volume 25 Issue 1 Pages 81-88
2019 Volume 25 Issue 1 Pages 81-88
The color of beer, which is a typical example of the Maillard or browning reaction, is mainly attributed to melanoidins. However, as melanoidins are heterogeneous polymers formed by the Maillard reaction, there is little data on the chemical structure of the components responsible for the color of beer. To obtain chemical information on the color components of beer, we here isolated a low-molecular-weight yellow pigment from black beer and identified it using instrumental analyses and an authentic sample. As a result, the pigment was identified as perlolyrine, which is a Maillard reaction product from tryptophan; however, its contribution to the total color of beer was very low. This pigment was present in various kinds of beer at the level of 3.2–14.0 µg/100 mL.
Beer shows a brilliant yellowish or gold color that is formed through the Maillard reaction. Pale beer is characterized by a pale-yellow color, while black beer shows dark brown or black. The major determinant of the color of beer is the malt. A normal malt used in the preparation of typical pale beer is dry-roasted at a temperature less than 100 °C, as the malt must maintain enzyme activity as well as good storage quality. On the other hand, special malts are used for the preparation of various kinds of beer including black beer, amber ale, stout etc. in addition to pale malt; these malts are heated to more than 100 °C and sometimes up to 225 °C during kilning to impart special flavor and color (Lewis and Young, 1995). During the heating process, the Maillard reaction occurs between reducing sugars and amino acids, peptides and/or proteins in malts (Carvalho et al., 2016). In addition to malting, the color or pigment of beer is partly formed during other heating process including mashing, boiling, and pasteurization (Fig. 1).
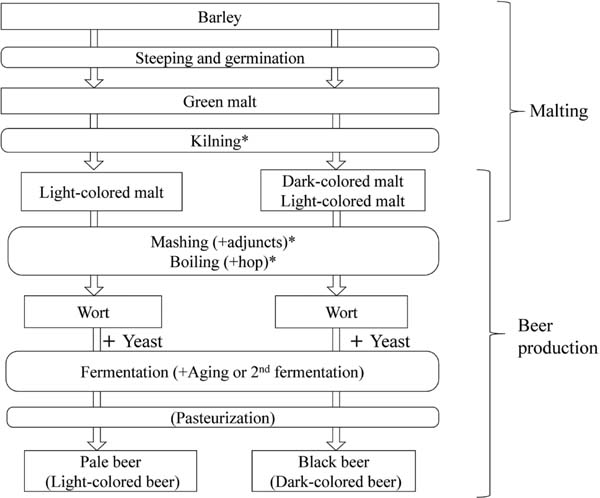
Overview of malting and beer production. Light-colored or normal malt is used for production of pale or light-colored beer, while dark-colored or special malt in addition to light-colored malt are used for production of black beer or dark-colored beer. * shows the process where color formation or browning mainly occurs by Maillard reaction.
The imparting of color to beer is mainly attributed to melanoidins. However, melanoidins are heterogeneous polymers formed by the Maillard reaction between carbonyl compounds such as sugars or their degradation products and amino compounds such as amino acids, peptides, and proteins. These compounds react with each other by various chemical reactions such as condensation, polymerization, degradation, cyclization etc. to form melanoidins. This complexity causes difficulties in the analysis of melanoidins (Echavarria et al., 2012), although some plausible chromophores of model melanoidins have been proposed (Hofmann, 1998; Hayase et al., 2006). In terms of the color or pigment of beer, some properties such as chromatographical characteristics and UV-Vis spectra (Kuntcheva and Obretenov, 1996) were examined; however, no clear data is available on the chemical structures. Although beer color is mainly attributed to melanoidins, and the contribution of individual low-molecular weight pigments to beer color seems to be low; we here searched for a low-molecular-weight pigment from beer to obtain chemical information regarding the color of beer. We observed that a peak having a maximum absorption wavelength in the visible region was present in the basic substance fraction of beer; thus, we described the isolation and identification of the pigment (BB1) and its distribution in various kinds of beer.
Materials Various kinds of beer were purchased from a retail shop in Tokyo between 2016–2018 and used for experiments.
Fractionation of beer samples Beer samples (two brands of pale beer; Kirin Co. (Tokyo, Japan) and Sapporo Breweries (Tokyo, Japan) and a brand of black beer (Sapporo Breweries)) were fractionated using ethyl acetate into neutral and acidic substance fraction, basic substance fraction, and water-soluble substance fraction. The pH of each beer sample was adjusted to less than 2 with 6 M HCl. Each sample was then extracted with a similar amount of ethyl acetate three times, before being concentrated in vacuo (acidic and neutral substance fraction). Then, the pH of the residual water layer was adjusted to 9–10 with 6 M NaOH. Each water layer was extracted with ethyl acetate three times, before being concentrated in vacuo (basic substance fraction). The residual water layer was designated as the water-soluble substance fraction. The volume of each fraction was arbitrarily adjusted to detect peaks using HPLC.
HPLC analysis Beer samples and their fractions were as described above. These beer samples and the fractions during purification procedures were analyzed with a reversed-phase HPLC system equipped with a photodiode-array detection (DAD) under the following conditions: system, Chromaster 5430 (Hitachi, Tokyo, Japan); column, Mightysil RP-18GP (4.6 mm i.d. x 250 mm, Kanto Chemical, Tokyo, Japan); eluent, solution A (acetic acid:water=0.1:100, v/v) and solution B (methanol:acetic acid:water=0.1:80:20; v/v/v)), 0 % B for 0–10 min and 0–100 % B (v/v) for 10–40 min with a linear gradient for beer samples, and 0–100 % B (v/v) for 0–30 min with a linear gradient for fractions during purification procedures; flow rate, 1.0 mL/min; wavelength, 230–500 nm. BB1 in beer samples and fractions during purification procedures were, respectively, detected at retention times of about 32 min and 22 min under these conditions.
Isolation of BB1 About 1 L of the black beer sample (Sapporo Breweries) was stirred for about 30 min to decrease the amount of foam, and then the pH was adjusted to less than 2 with 6 M HCl. The beer sample was washed with a similar amount of ethyl acetate three times, and then the pH of the residual water layer was adjusted to 9–10 with 6 M NaOH. BB1 was extracted with ethyl acetate three times. The combined ethyl acetate layer was concentrated in vacuo, and about 60 mg of brown paste was obtained. These procedures were repeated ten times. The combined paste from 5 L of black beer was dissolved into 50 mL of a mixture of methanol, acetic acid, and water (40:0.1:60; v/v/v), which was applied to a Chromatorex ODS column (i.d. 20 mm × 100 mm; Fuji Silycia Chemical, Kasugai, Japan). The column was developed with a mixture of methanol, acetic acid, and water (40:0.1:60 and 80:0.1:20; v/v/v) and methanol, successively. Fractions containing BB1 (methanol, acetic acid, and water = 80:0.1:20) were combined and concentrated in vacuo. The obtained brown paste was dissolved in methanol and applied to preparative HPLC under the following conditions: pump, L-6000 (Hitachi); column, YMC-Actus Triart C18 (20 mm i.d. × 250 mm, YMC, Kyoto, Japan); eluent, methanol:acetic acid:water = 50:0.1:50; v/v/v); flow rate, 9.99 mL/min; detector, L-4200 (Hitachi); wavelength for detection, 400 nm. The fraction from the peak at a retention time of about 19 min was collected. The combined fractions were concentrated in vacuo, resulting in a yellow paste (less than 1 mg) from about 10 L of dark beer sample.
Instrumental analyses Spectroscopic measurements were conducted using the following instruments: spectrophotometer (Multispec-1500, Shimadzu, Kyoto, Japan), NMR (Bruker Avance 600, Bruker Biospin, Karlsruhe, Germany), and MS (Triple TOF 4600, AB Sciex, Foster City, CA, USA).
Physicochemical properties of BB1 MS (m/z): 265.0961 [M+H] +, calcd. for C16H13N2O2 265.0974; 263.0821 [M-H]−, calcd. for C16H11N2O2 263.0816. 1H- and 13C-NMR data are summarized in Fig. 4.
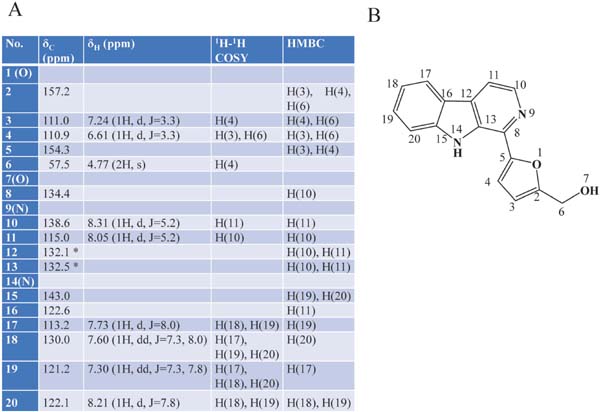
NMR spectral data of BB1 (perlolyrine) in MeOD (A), and its structure and numbering (B). *, exchangeable.
Quantification of perlolyrine (BB1) in beer Each beer sample was stirred for about 30 min to remove carbon dioxide, before adjusting the pH to 9–10. BB1 was then extracted from 100 mL of each sample with ethyl acetate. The ethyl acetate layer was concentrated in vacuo, and was dissolved in 2 mL of methanol. BB1 was measured using HPLC with an external standard method under the following conditions: pump, L-6000 (Hitachi); column, Mightysil RP-18 GP (4.6 mm i.d. × 25 mm, Kanto Chemical); eluent, solution A (methanol: acetic acid:water =20:0.1:80; v/v/v) and solution B (methanol: acetic acid:water =60:0.1:40; v/v/v), 0–100 % B (v/v) for 30 min with a linear gradient; flow rate, 1.0 mL/min; detector, L-4200 (Hitachi); wavelength for detection, 405 nm. BB1 was detected at a retention time of about 19 min under this condition. We found that the detection limit of BB1 was less than 1 µg/100 mL of beer.
Preparation of authentic perlolyrine Perlolyrine was prepared according to the method of Jeffreys (1970) and Tang et al. (1998), with some modifications. Briefly, 20 mM tryptophan and 20 mM 5-acetoxymethyl-2-formylfuran (Sigma-Aldrich Japan, Tokyo) was dissolved in 100 mL acetic acid, which was heated in boiling water for 10 min. About 160 mL of 1.3 % H2O2 solution was added to the solution, which was further heated for 10 min. The basic substance fraction was prepared from the reaction solution using ethyl acetate, prior to purification of perlolyrine using Chromatorex ODS and HPLC as described above. About 220 mg of perlolyrine was obtained as a yellow powder. NMR data were the same as shown in Fig. 4A.
Evaluation of color activity and color contribution of perlolyrine Color activity and color contribution were estimated with the color dilution method (Hofmann, 1998). Perlolyrine dissolved in a 0.5 M sodium acetate buffer (pH 4.2) and beer samples were successively diluted with the buffer, and each aliquot (100 µL) was placed into a 96-well microplate. Individual detection limits for perlolyrine and beer were visually estimated with the triangle test, using ten panelists. The color activity (unit) of perlolyrine in a beer sample was calculated as [perlolyrine concentration (µg/mL)]/[its detection limit (µg/mL)]. The contribution of perlolyrine to total color (%) of the sample was calculated by [color activity of perlolyrine in beer]/[detection limit of beer (dilution ratio)] × 100.
Pigment in the basic substance fraction of beer First, samples of pale and black beers were applied to ODS-HPLC equipped with DAD detection; however, no peaks showing a clear absorption maximum in the visible region could be detected. It is possible that melanoidins as the major pigment prevented the detection of small amounts of low-molecular-weight pigments in HPLC. Then, we fractionated a black beer into the neutral or acidic substance, the basic substance, and the water-soluble substance fractions using ethyl acetate. Melanoidins are generally thought to be transferred to the water-soluble substance fraction. Each fraction was analyzed with DAD-HPLC (Fig. 2). As a result, a peak (BB1) showing a specific UV-Vis spectrum (Fig. 2D) was detected in the basic substance fraction of a dark beer (Fig. 2C). We then examined whether this peak appeared in the extract of other brands of pale and black beers. All the basic substance fractions from the examined beer samples showed the same peak (data not shown). We determined that BB1was a common pigment present in beer, and aimed to isolate and identify this pigment.
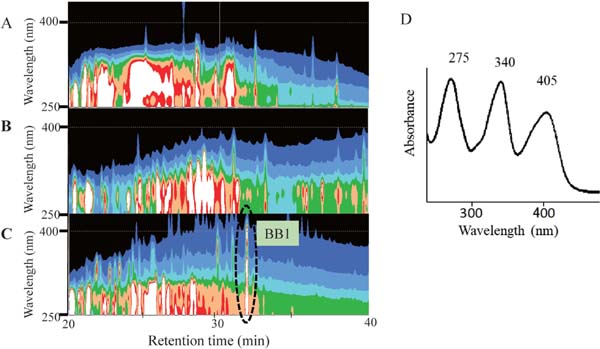
Searching for a low-molecular-weight pigment in beer using DAD-HPLC. Contour maps of black beer (A), its acidic and neutral substance fraction (B) and its basic substance fraction (C), and UV-Vis spectrum of BB1 in C (D). Intensities of absorption in contour maps are divided into eight equal parts as white (highest), red, orange, green, light blue, blue, indigo blue, and black (lowest).
Identification of a low-molecular-weight pigment (BB1) in dark beer As black beer contained more pigment than the examined pale beer, we employed black beer for the isolation of BB1. After preparing the basic substance fraction, we isolated BB1 from the fraction using the chromatographic procedures described in the Materials and Methods. Less than 1 mg of yellow paste was obtained from 10 L of dark beer.
Figure 3 shows the UV-Vis and fluorescence spectra of BB1. BB1 showed three absorption maxima at 275, 340, and 405 nm in an acidic solution, meaning that this compound is yellow in beer, as the pH of beer is about 4.2 and weakly acidic. The absorption spectra showed a hypsochromic shift in the basic solution, suggesting the presence of a dissociation group containing nitrogen atom. This pigment showed blue fluorescence.
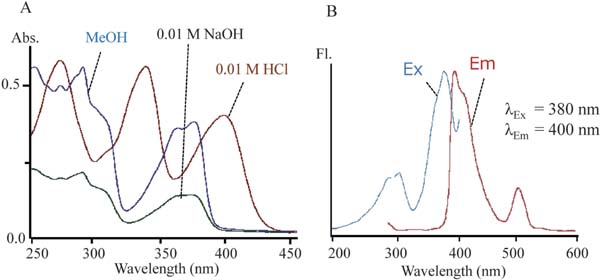
UV-Vis (A) and fluorescence (B) spectra of BB1. BBI was dissolved in 0.01 M HCl, 0.01 M NaOH and methanol for UV-Vis spectra and dissolved in methanol for fluoresce spectra.
The mass spectrum of BB1 showed its molecular weight, molecular formula, and desaturation degree were 265, C16H12O2N2, and 12, respectively. The unique UV-Vis spectra, fluorescence spectra, and high degree of unsaturation suggest this compound is a heterocyclic aromatic compound.
The NMR data are summarized in Fig. 4. The NMR analyses showed 16 kinds of carbons corresponding to the molecular formula. The heteronuclear single quantum correlation (HSQC) analysis showed that a carbon (δC 57.5), eight olefinic carbons (δC 110.9, 111.0, 113.2, 115.0, 121.2, 122.2, 130.0, and138.6), and the residual seven olefinic carbons (δC 122.6, 132.1, 132.5, 134.4, 143.0, 154.3, and 157.2) were, respectively, a methylene group, methine groups, and quaternary. The chemical shifts of the methylene group (δC 57.5 and δH 4.77) suggest that it is connected to an oxygen atom or hydroxy group. Further, the heteronuclear multiple bond correlation (HMBC) analysis showed that the singlet protons of this methylene group were correlated with the quaternary carbon at δC 157.2 and the methine carbons at δC 111.0 carrying a proton at δH 7.24 and at δC 110.9 carrying a proton at δH 6.61. The proton at δH 6.61 was coupled with the proton at δH 7.24. Further, the HMBC analysis showed that the proton at δH 7.24 was correlated with a quaternary carbon at δC 154.3. These connections suggest the presence of a 5-hydroxymethylfurfuryl group (Fig. 5A). Moreover, the 1H-1H-correlation spectroscopy (COSY) and HMBC data showed a partial structure of =C-CH=CH-CH=CH-C= (Fig. 5A). The residual two protons (δH 8.05 and 8.31) of two methine groups were coupled to each other. In addition to these substructures, molecular formula, chemical shift, HMBC and nuclear Overhauser effect (NOE) spectroscopy (NOESY) data suggested two plausible structures for BB1 (Fig. 5B). In the two structures, structure X was reported as perlolyrine by Jeffreys (1970). The reported physicochemical data, including UV-Vis absorption spectra and NMR, were very similar to those of BB1. To confirm this, we prepared an authentic perlolyrine from tryptophan and methyl ester of 5-hydroxymethylfurfural, and then compared the isolated BB1 and the authentic perlolyrine. As a result, their retention times on DAD-HPLC, UV-Vis spectra, and NMR data coincided completely. Thus, we identified BB1 as perlolyrine ([5-(9H-pyrido[3,4-b]indol-1-yl)furan-2-yl]methanol; Fig. 4B).
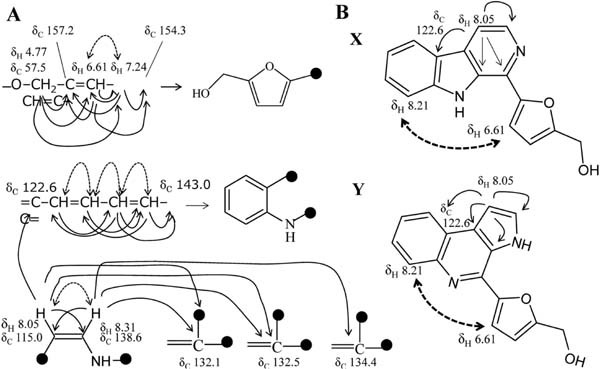
Partial structures (A) and plausible structures (B) of BB1. In A, single arrows and dotted two direction arrows show HMBC and 1H-1H coupling, respectively. In B, single arrows and dotted two direction arrows show HMBC and NOE, respectively.
Perlolyrine was first isolated from perennial rye-grass (Lolium perenne) as an alkaloid showing fluorescence (Jeffreys, 1970), and subsequently isolated from Japanese sake as a yellowish green fluorescent compound (Oba et al., 1978) and from dangshen (Codonopsis pilosula), a traditional tonic medicine in China (Tun et al., 1988). This compound was also isolated from soy sauce as a yellow and weakly mutagenic β-carboline derivative (Nakatsuka et al., 1986; Oshita et al., 1991). Perlolyrine is known to have biological or physiological activities such as a phase-Ⅱ enzyme induction in vitro (Li et al., 2011), antiproliferative activity against human stomach cancer cell lines (Lee et al., 2016), and transient receptor potential vanilloid 1 activation in vitro and taste modification in vivo (Oshida et al., 2017). Perlolyrine is also known to be formed from tryptophan and 5-hydroxymethylfurfural or sugars through the Maillard reaction (Oshita et al., 1993; Lee et al., 2016).
Distribution of perlolyrine in various kinds of beer To the best of our knowledge, this is the first identification of perlolyrine in beer. We then examined the amount of perlolyrine in various kinds of retail beer (Fig. 6). The 10 kinds of beer samples examined contained 3.2–14.0 µg/100 mL of perlolyrine (average ± standard deviation; 7.1±3.7 µg/100 mL). Among them, seven kinds of pale or light-colored beer (A-G in Fig. 6) contained 3.2–8.0 µg/100 mL (5.5±1.5 µg/100 mL) of perlolyrine, while three kinds of black or dark-colored beer (H-J in Fig. 6) contained 4.8–14.0 µg/100 mL (10.7± 5.1 µg/100 mL) of perlolyrine. Although black beer contained more perlolyrine than pale beer, the difference was not large (7.1 vs 10.7 µg/100 mL). Beer-like alcoholic beverages contained various amount of perlolyrine (0–22.9 µg/100 mL).
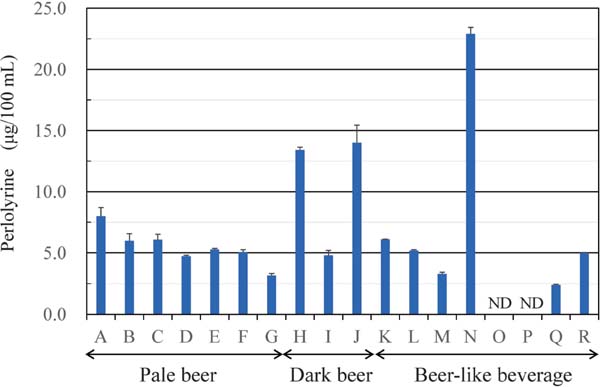
Perlolyrine in various kinds of retail beer and bee-like alcoholic beverage. A-G are pale or light-colored beer. H-J are black or dark-colored beer. K-N are light-colored beer-like alcoholic beverage made of less than 25 % malt as raw materials. O-Q are light-colored non-malt beer-like alcoholic beverage. R is dark-colored beer-like alcoholic beverage made of less than 25 % malt as raw materials. ND, not detected. (n=3).
Perlolyrine is formed from tryptophan and 5-hydoxymethylfurfural by the Maillard reaction (Oshita et al., 1993; Lee et al., 2016). Figure 7 shows a schematic of perlolyrine formation during beer production. During mashing, tryptophan is formed from malt peptides or proteins, while 5-hydoxymethylfurfural is formed from glucose or maltose derived from starch. The two substrates react with each other to form perlolyrine during mashing, boiling and other subsequent processes, including fermentation, pasteurization, and storage. We aim to further examine the effect of mashing and boiling conditions on the formation of perlolyrine, as we did not detect perlolyrine in malt extracts in a preliminary experiment (data not shown).
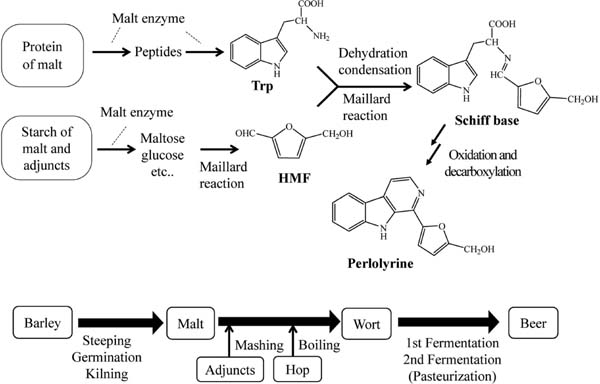
Formation scheme of perlolyrine during beer production. Proteins of malt and other cereal are hydrolyzed to produce tryptophan by malt proteinase and peptidase during mashing. At the same time, starch of malt and other cereals are hydrolyzed to produce maltose and glucose, which are converted to 5-hydroxymethylfurfural (HMF) during boiling of mash by the Maillard reaction. Tryptophan and HMF in wort are condensed to form Schiff base, from which perlolyrine is formed by oxidation and decarboxylation during wort preparation, 1st fermentation, 2nd fermentation, pasteurization, and storage.
The absorbance of pale beer at 400 nm was about 0.5, while the absorbance derived from perlolyrine at 400 nm was estimated to be about 0.002 according to its content (0.055 µg/mL beer). This result indicated that perlolyrine represented about 0.4 % (0.002/0.5 × 100 = 0.4) of the absorbance at 400 nm of pale beer. Next, the sensory contribution of perlolyrine to the overall color of pale beer was estimated using the color dilution method (Hoffman, 1998). A sodium acetate buffer (pH 4.2) was used as the solvent here, as the pH of beer is about 4.2. The detection limit for the visual estimation of perlolyrine was 50 µg/mL. On the other hand, a pale beer sample contained 0.051 µg /mL of perlolyrine, and the color of this beer was visually detected at a 2-fold dilution. The rate of color contribution of perlolyrine in this beer was calculated to be only 0.05 % according to this data (0.051/50/2×100 = 0.051). This estimation means that the contribution of perlolyrine to the total color of beer is very low and that the major contributors to beer color are melanoidins, heterogenous high-molecular-weight pigments. However, we must acknowledge that the recognition of color is cumulative, and even if the individual contribution of a pigment is low, the total or cumulative contribution will be meaningful in the context of the multifarious pigments in beer.
In conclusion, we isolated a low-molecular-weight Maillard pigment from beer and identified it as perlolyrine. This is the first identification of a Maillard pigment in beer; however, its contribution to the total color of beer is very small.
Acknowledgements This study was supported by a Grant-in-Aid for Scientific Research (B) [grant number 17H01958] from the Japan Society for the Promotion of Science.