2019 Volume 25 Issue 1 Pages 97-106
2019 Volume 25 Issue 1 Pages 97-106
Protein oxidation can alter the structure of myofibrillar proteins (MPs), which further affect the MPs gelling properties. Ionic strength can influence MPs oxidation. The objective of the study was to evaluate changes in structure and gel properties of MPs induced by hydroxyl radicals (•OH) under certain ionic strength and pH. MPs were very susceptible to •OH attack under 0.5 M NaCl (pH 6.25) due to their swelling structure, resulting in higher protein oxidation level. Oxidative alternation of MPs structure had a significant influence on gel properties and microstructure. The maximum of storage modulus (G′) during the cooling stage was observed at 1.0 mM H2O2. Results of SEM confirmed that the gel had a more compact and dense structure at 1.0 mM H2O2. The results demonstrated that mild oxidative modification (H2O2 ≤ 1.0 mM) induced MPs aggregation, which was benefit to the formation of gel elastic network. However, further oxidative modification (2.5 mM ≤ H2O2 ≤ 10.0 mM) led to an excessively cross-linking of MPs, which made a negative effect on gel properties.
Gel formation or gelation is one of the most important functional properties of muscle protein which affects the textural characteristics and palatability of processed muscle foods, such as Cantonese sausage, Frankfurt sausage, and boneless ham (Sun et al., 2011b; Sun and Holley, 2011; Wu et al., 2010). In different protein groups, myofibrillar proteins (MPs), contributing 55–65 % of total muscle protein, are largely responsible for the structural characteristics of meat products. In general, muscle protein gels with a fine network can effectively bind meat and fat particles, while gels with a coarse network exhibit poor binding capacity (Xiong et al., 2010). There is no substitute for the myofibrillar protein myosin in gel formation by proteins from a wide variety of animal and fish species.
Considerable research on the thermal gelation ability of MPs has been well documented. Gelling properties are dependent on several intrinsic (such as meat species, types of muscles and fibers, etc.) and extrinsic factors (such as pH, temperature, ionic strength, rates of heating, etc.) (Boyer et al., 1996; Feng et al., 2018; Sun and Holley, 2011; Yu et al., 2017). Among these factors, ionic strength plays a key role in gel formation due to their ability to dissolve MPs. Sodium chloride is the salt commonly used in processed meat products and affects flavor, texture and shelf life of meat products (Xing et al., 2017). An aggregated-type gel is formed from monomeric myosin (at >0.5 ionic strength or 0.5 M NaCl), while a strand-type gel is formed from filamentous myosin (at 0.2 to 0.3 M NaCl) (Xiong et al., 2010). The binding of Cl_ to actomyosin can enhance the electrostatic repulsion of MPs, which makes a positive effect on well expansion of MPs fiber (Li et al., 2013).
Protein oxidation is a significant factor that affects the gelation properties during meat processing. Reactive oxygen species (ROS), the main initiators of protein oxidation, can be generated during meat product manufacturing process (Park et al., 2006; Wang et al., 2017a). MPs in processed meat products unavoidably undergo attack from ROS. The amino acid side chains and the structure of protein are greatly involved when oxidation occurs, mainly resulting in the formation of carbonyls, inter- and intra-molecular cross-linking through the formation of disulfide bonds, dityrosine, Schiff bases, and fragmentation of the peptide backbone etc. (Grune et al., 2004), which ultimately result in gel texture changes (Sun et al., 2011a; Wang et al., 2017a). Changes in the secondary and tertiary structure of MPs due to oxidation can lead to the unfolding of MPs structure, which further improves protein– protein interactions, and thus affect their gel properties (Park et al., 2006; Wang et al., 2017b; Zhou et al., 2014a). Therefore effect of oxidative stress under certain ionic strength on gel-forming ability of MPs is still worthy of further investigation. Liu et al. (2011) found that salt can promote MPs oxidation. Under normal meat processing ionic strength and pH (pH 6, NaCl 0.6 M), mild oxidation could promote the gel formation (Xiong et al., 2010). Based on consumers have become more aware of the relationship between sodium and health, the demand for a variety of low salt meat products has increased. Therefore, ionic strength (NaCl 0.5 M, pH 6.25) and a hydroxyl radical system (generated by Fe3+-Vc-H2O2) were used in this work. The physicochemical and structural changes of MPs and the gel properties were investigated to elucidate the structure-modifying effect of oxidative stress under these conditions.
2.1. Preparation of MPs Longissimus dorsi from pork carcasses was purchased from a local commercial abattoir (Guangzhou, China) and the pigs were slaughtered about 6 months of age following standard industrial procedures. Fat was trimmed away and muscle was cut into cubes, and then used for the MPs preparation. MPs were extracted with four volumes of phosphate buffer solution (PBS) according to the method of Xiong et al. (2010). PBS (10 mM) at pH 7.0 containing 0.1 M NaCl, 2 mM MgCl2 and 1 mM EGTA was used as extraction buffer and centrifugation conditions were 2000× g for 15 min at 4 °C. After the PBS buffer washing, precipitate was washed three more times with 0.1 M NaCl solution, in the final washing, the pH of protein suspension was adjusted to 6 using 0.1 M HCl (to closely simulate the pH condition in processed meats). The final MPs pellet was suspended in PIPES buffer (15 mM, pH 6.25). Protein concentration of the MPs pellet was measured by the Biuret method using bovine serum albumin (Sigma Chemical Co., St. Louis, Mo., USA).
2.2. Incubation of MPs with NaCl and hydroxyl radicals MPs were oxidized according to the procedure described by Xiong et al. (2009). MPs suspension (30 mg/mL) was incubated at 4 °C for 24 h in PIPES buffer (15 mM, pH 6.25) containing 0.5 M NaCl with an iron-catalyzed oxidizing system that generates hydroxyl radicals. The hydroxyl radicals were produced by a 10 µM FeCl3/100 µM ascorbic acid solution with H2O2 at various concentrations (0–10 mM). The sample without H2O2 was chosen as the control. To terminate further oxidation, EDTA (1 mM final concentration) was then added. The treatment samples were divided into two portions, one portion was used to measure the physicochemical property changes of MPs. Another was used to analyze rheological and gel properties changes of MPs.
2.3. Measurement of oxidative changes The concentration of different MPs samples were diluted into 5 mg/mL with PIPES buffers before physicochemical properties testing.
2.3.1. Determination of carbonyl and sulfhydryls (SH) content Protein carbonyls in MPs were determined according to the procedure described by Sun et al. (2011a) with some modifications. Briefly, two aliquots of 0.5 mL MPs suspension were transferred into centrifuge tube. One portion was treated with 2 mL 0.2 % (w/v) DNPH in 2 M HCl, another mixed with an equal volume of 2 M HCl acted as a control. And then samples were incubated for 1 h at room temperature (25 °C) avoiding light. After completing the incubation, added 625 µL 50 % (w/v) trichloroacetic acid (TCA) and mixed well with XW-80A Vortex mixer (Shanghai Jingke Instrument Co. Ltd., Shanghai, China), and then centrifuged (2000×g, 15 min, 4 °C). Precipitation were recovered and then washed three more times with 3 mL of ethanol:ethyl acetate (1:1, v/v) to eliminate free DNPH. The final MPs pellets were dissolved in 3 mL of 6 M guanidine HCl with PIPES buffer (15 mM, pH 6.25) and incubated with a water bath for 30 min at 37 °C. The control samples was used to estimated protein concentration at 280 nm and BSA in 6 M guanidine played as a standard. The carbonyl concentration was measured on the treated samples by using the absorption of 21.0 mM−1cm−1 at 370 nm against the HCl control for protein hydrazones.
The SH content was determined by a modification of Ellman's method using 2,2′-dithiobis (5-nitropyridine) (DTNP) according to Wang et al. (2017b). In this work, PIPES buffer (15 mM, pH 6.25) was used as a blank instead of PBS. Results were expressed in micromoles of SH per gram of protein.
2.3.2. SDS-polyacrylamide gel electrophoresis (SDS-PAGE) SDS-PAGE was subjected to evaluate MPs changes during oxidation according to Wang et al. (2016) with a 5 % polyacrylamide stacking gel and a 12 % polyacrylamide separating gel. Briefly, MPs (5 mg/mL) were mixed with an equal volume of SDS-PAGE sample buffer with or without 10 % β-mercaptoethanol (β-ME). The mixture was heated in boiling water (100 °C) for 3 min, centrifuged (10,000×g, 10 min, 4 °C). An aliquot of 10 µL of samples was loaded. After running, the gels were stained for 1 h with 0.1 % Coomassie brilliant blue R250 in 50 % methanol and 6.8 % acetic acid and subsequently destained with 5 % methanol and 7.5 % acetic acid.
2.3.3 Determination of droplet size distribution Particle size distributions of MPs dispersed in PIPES buffer (15 mM, pH 6.25) were determined using a Malvern Mastersizer 2000 (Malvern Instruments Ltd., Malvern, Worcestershire, UK) equipped with a He–Ne laser (623 nm) according to the method of Sun et al. (2014). The relative refractive index and absorption were set as 1.414 and 0.001, respectively. The device was programmed to count and calculate the size of particles with diameters of 0.02–2000 µm.
2.3.4. Determination of protein surface hydrophobicity The hydrophobicity of MPs was determined using the hydrophobic chromophore BPB according to Sun et al. (2013) with some modifications. To 1 mL of MPs suspension, 200 µL of 1 mg/mL BPB (in distilled water) was added and mixed well. A control, without MPs, was prepared by addition of 200 µL of 1 mg/mL BPB (in distilled water) to 1 mL of PIPES buffer (15 mM, pH 6.25). Samples and control were kept under agitation, at room temperature, for 10 min. The samples centrifuged at 4000×g for 15 min (4 °C), and then diluted the supernatant 10 times with PIPES buffer before absorbance measured at 595 nm. The amount of BPB bound, given by the following formula, is used as an index of hydrophobicity:
 |
2.4. Gelation and tests for gel properties
2.4.1 Preparation of MPs gel Eight grams of sols were transferred into glass vials (25 mm inner diameter) and then heated from 25 to 80 °C at 1 °C/min increments in a water bath (kept for 5 min when temperature reached at 53 °C). After reaching the final temperature, gels were immediately chilled in the ice slurry and stored at 4 °C overnight before any measurements.
2.4.2 MPs gel strength Gel texture was measured according to the method of Zhou et al. (2014a) using a cylinder measuring probe (P 0.5SS) attached to a TA.TX2 texture analyzer (Stable Micro System, Surrey, UK) at a constant probe speed of 1.0 mm/min at room temperature (25 ± 1 °C). Gel strength is defined as the initial force required to disrupting the gels. All samples were prepared in triplicate.
2.4.3 Water-holding capacity (WHC) measurement Gels (3 g) were centrifuged at 6000×g for 15 min (4 °C). The WHC (%) was expressed as the final weight of the pellet (g) after centrifugation occupied the percentage of the weight before centrifuging.
2.4.4. Dynamic rheology measurement The viscoelastic characteristics of the MPs during thermal gelation were measured with a Haake MARS Ⅲ Rheometer (Thermo Electron GmbH, Karlsruhe, Germany) with a parallel plate sensor (diameter 35 mm) in oscillatory mode. The specific method described by Wu et al. (2009) with some modifications. In order to be consistent with formation of thermal induced MPs gel, the protein concentration of the samples used for the rheological test was 30 mg/mL in this work. Storage modulus (G′) was recorded to demonstrate viscoelastic changes during the sol-to-gel transition.
2.4.5 Microstructure The internal microstructure of the gel were measured with scanning electron microscopy (SEM) (S-3400N Hitachi High Technologies Corp., Tokyo, Japan) according to Zhou et al. (2014a) with some modification. Briefly, the scan voltage is 15 kV and the magnification is ×1000. MPs gels were cut into 3×3×3 mm3 cubes from different areas of each gel and snap-frozen in liquid N2 before transverse slices were obtained and applied to the SEM. Fresh samples were rapidly immersed into liquid nitrogen slush, which was freshly filled to prevent the presence of particulates that may provide nucleation for the growth of ice crystals.
2.5. Statistical analysis Statistical calculation was investigated using the statistical package SPSS 11.5 (SPSS Inc., Chicago, IL, USA) for one-way ANOVA. Student- Newman-Keuls test was used for comparison of mean values among three treatments using a level of significance of 5 %. Triplicate preparations of MPs were carried out to confirm the accordance and all data showed were from the same preparation. For gel determinations, triplicate measurements were made on three different gels that were prepared for each treatment. Data were expressed as means ± standard deviations of triplicate determinations.
3.1 Effect of oxidative modification on physicochemical changes of MPs Amino acids with NH2 or NH groups (lysine, histidine, arginine) on protein side chains are sensitive to •OH attacking and then transferred into carbonyl groups (Morzel et al., 2006). Carbonyl content can be used as an indicator of protein oxidative damage (Estevez et al., 2005). The carbonyl content of all samples is presented in Table 1. In this work, carbonyl content of control was 1.27 nmol/mg protein. The carbonyl content increased (p < 0.05) with the addition of H2O2 up to 1.0 mM and then reduced gradually (p < 0.05), the alteration trend was consistent with Park et al. (2006). The carbonyl contents in this work were higher (p < 0.05) than our founding in oxidative system applying no NaCl at the same amount of H2O2 addition (Sun et al., 2013). These results indicated that high salt concentration could promote oxidation of MPs (Liu et al., 2011). MPs incubated with high concentration of NaCl (final concentration was 0.5 M) might tend to swell profusely (Feng et al., 2018), resulting in the swelling structure of MPs became more sensitive to •OH attack. But further addition of H2O2 facilitated the formation of protein-protein cross-links (Stadtman and Levine, 2000; Zhou et al., 2014a) and carbonyl-amine cross-links via carbonyls attack nucleophiles (such as ε-NH2 of lysine), which attributed to the loss of carbonyl contents (Li et al., 2012).
| Concentration of H2O2/mM | Carbonyl/(nmol•mg−1 protein) | SH / (nmol•mg−1 protein) | Surface hydrophobicity / (µg•mg−1 protein) |
|---|---|---|---|
| 0.0 | 1.27±0.16a | 71.28±0.27e | 38.10±0.88a |
| 0.1 | 1.61±0.05a | 70.89±0.35e | 99.62 ±2.38d |
| 0.5 | 2.75±0.12b | 56.45±0.39b | 101.33±6.27d |
| 1.0 | 7.49±0.48d | 63.56±1.29d | 131.36±4.15e |
| 2.5 | 5.40±0.12c | 61.95±1.08d | 91.97±1.18c |
| 5.0 | 2.46±0.21b | 60.28±0.58c | 65.83±1.28b |
| 10.0 | 1.61±0.12a | 45.11±0.33a | 61.66±3.57b |
a–e Means in the same column with different superscript letters are significantly different (p < 0.05)
The contents of SH of the control and oxidized MPs are also shown in Table 1. The SH content of the control was 71.28 nmol/mg protein, similar to that reported in the previous studies (Wang et al., 2017b; Zhou et al., 2014b). Compared to the control, treatment with low concentrations of H2O2 (below 0.1 mM) resulted in no significant (p > 0.05) change in the SH content. However, a marked decrease in SH content was found with further addition of H2O2 (0.5–10.0 mM).
As shown in Table 1, surface hydrophobicity of the control was 38.10 µg, similar to that reported by Zhou et al. (2015). Compared to the control, protein surface hydrophobicity increased remarkably (p < 0.05) with the increasing of H2O2 concentration (H2O2 ≤ 1.0 mM), and reached the maximum (131.36 µg) at 1.0 mM H2O2 treatment to MPs. However, upon further addition of oxidation agent (H2O2 ≥ 2.5 mM), a downward trend (p < 0.05) in surface hydrophobicity was observed. Mild oxidation (H2O2 ≤ 1.0 mM) induced the exposure of nonpolar amino acids (or hydrophobic amino acids) to the protein surface, which are buried inside in native status, resulting in the increasing of surface hydrophobicity. But protein aggregation formed at high concentration of H2O2 partially shielded the exposure of nonpolar amino acids to the protein surface due to the increased protein-protein interaction. These results were consistent with previous studies (Li et al., 2012; Zhou et al., 2015).
3.2 SDS-PAGE electrophoresis Protein–protein interaction and aggregation prior to heating play a crucial role in the ensuing gel network formation (Zhou et al., 2014a). The aggregation and structural changes of modified MPs were analyzed by SDS-PAGE. As shown in Fig. 1A, incubation with 0.1–1.0 mM H2O2 caused a gradual disappearance of myosin heavy chain (MHC). The bands of MHC were barely attenuated, but were more diffused compared with the control when incubation with 2.5–5.0 mM H2O2. It might be due to the simultaneous aggregation and degradation of MPs caused by protein oxidation. Significant amounts of MHC were lost with large polymer appeared in the top stack gel when the concentration of H2O2 reached 10.0 mM. However, actin (AC) was stable to •OH attack throughout the oxidation process. Moreover, MHC in all samples recovered in the presence of β-ME (Fig. 1B), suggesting that most cross-linking originated from myosin was through disulfide bond, and eventually leading to the reducing of myosin content and changes in myosin structure.

SDS–PAGE without (A) and with (B) β-ME of MPs oxidized by different concentration of H2O2. Std: standard, MW: molecular weight, Ag: aggregates, MHC: myosin heavy chain, AC: actin.
3.3 Determination of particle size distribution The particle size distribution can reflect the MPs structural changes (Sun et al., 2013). As shown in Fig 2, compared to the control, mild oxidizing agent treatment (H2O2 ≤ 1.0 mM) induced the MPs particle size distribution moving to the direction of small particle size by a concentration-dependent manner. However, the particle size distribution shifted to large particle size with the addition of oxidizing agent increased sequentially (H2O2 ≥ 2.5 mM). The MPs particle size distribution became unconcentrated and the peak of particle size distributions moving to large particles obviously when the concentration of H2O2 reached to 10.0 mM.

Particle size distributions of MPs oxidized by different concentration of H2O2 at 0.5 M NaCl.
The big particles corresponded to lengthy myofibril fragments. The decrease in particle size after mild oxidation might be explained by the formation of inter- or intramolecular cross-links and protein aggregates (Promeyrat et al., 2010; van der Linden and Venema, 2007). Mild oxidation induced the shift of myosin cross-linking from head–head association to tail–tail aggregation (Xiong et al., 2010). These aggregates look like spherical aggregates or a disorganised stack of fibres (Promeyrat et al., 2010). On the contrary, excessive oxidizing agent addition induced MPs coalescence and protein aggregations formation. The structure of these aggregated proteins may also be linear up to micron-scale, which may in turn form super-structures like bundles (van der Linden and Venema, 2007). Hydrophobic interactions played an important role in food protein aggregation. In this work, a negative correlation was observed between the particle size and the protein surface hydrophobicity (Table 1). The result was also consistent with the analysis of SDS-PAGE (the polymer appeared in the top stack gel) in Fig.1.
3.4 Gel strength and WHC analysis Gel strength and WHC played an important role in the controlling of gel quality (Horita et al., 2014). As shown in Fig. 3, a biphasic evolution of gel strength and WHC value was observed with increasing addition of H2O2. The maximum values of gel strength and WHC (2.15 N and 89.98 %, respectively) occurred when MPs treated with 1.0 mM of H2O2. Protein cross-linking and subsequent formation of gel network upon heat treatments were promoted by MPs modification with mild oxidation (Wang et al., 2017a; Xiong et al., 2010). Gel strength and WHC value exhibited the minimum values when treated with 10.0 mM H2O2, indicating that excessive oxidation caused negative effect on water-holding capacities and gel texture (Utrera and Estevez, 2012).

Effect of incubation with different concentration of H2O2 at 0.5 M NaCl on gel strength and WHC of MPs gel. Bars with different letters indicate significant differences (p < 0.05) among oxidation levels.
As aforementioned, in the presence of 0.5 M NaCl, mild oxidation modification promoted MPs aggregation, which was accompanied by decreasing of space resistance. Soluble MPs aggregations formed by protein–protein orderly interaction, which were finally benefit to the formation of regular gel matrix during heating process (Xiong et al., 2010; Yu et al., 2017). Structural integrity and content of myosin played a dominant role in the formation of gel matrix (An et al., 1996). Excessive oxidative modifications of MPs caused myosin degradation, MPs structural damage, and insoluble protein aggregation. All these contributed to an inferior gel network formation.
3.5 Dynamic rheological properties of MPs Dynamic rheological testing was used to monitor protein–protein interactions and viscoelastic network formation during the sol-to-gel transition. The rheograms of the MPs samples during heating and cooling are presented in Fig. 4. The elastic storage modulus (G′) is an important rheological parameter used to characterize the gel elasticity and structural network (Xiong et al., 2010). During the heating process from 25 to 85 °C (Fig. 4A), the control exhibited a typical rheological pattern of the transformation of a tacky protein sol to a viscoelastic gel network. The G′ of the control started to increase at 43.6 °C leading to a transition that peaked at 53 °C and a G′ trough at 58 °C followed by a steady increase over the 58 to 73 °C. The transition peak existed at 58 °C related to the cross-linking of denatured myosin head (Egelandsdal et al., 1986).
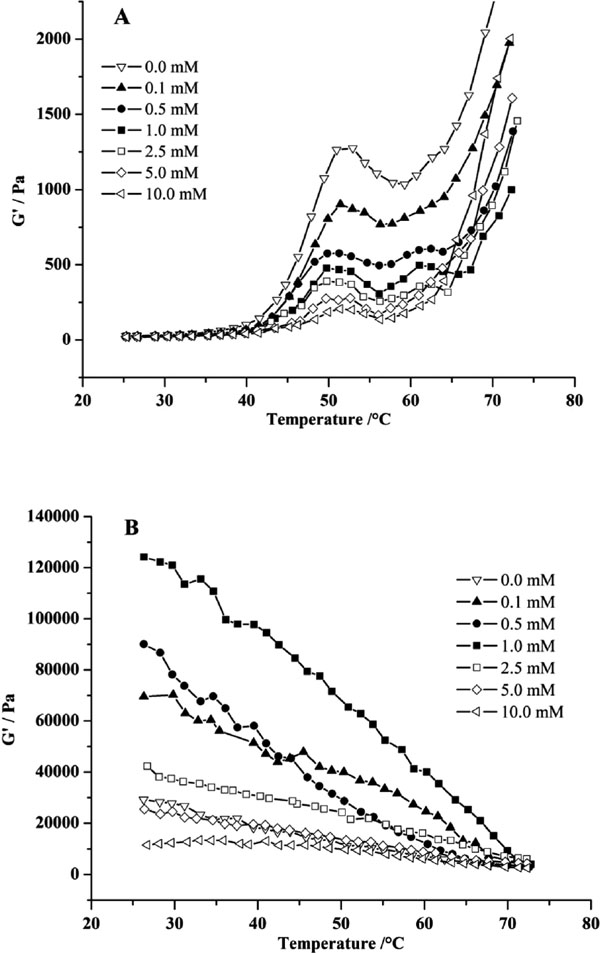
Storage modulus (G′) of MPs gel during heating (A) and cooling (B) stage at different concentration of H2O2 at 0.5 M NaCl.
Compared to the control, the rheological pattern changed after oxidation and the change was also dose-dependent. Induction with H2O2 lowered the transition peak temperature of G′ by about 2 °C, which might be due to the formation of more cross-links at lower temperatures oriented from preheating and relevant oxidation effect (Zhou et al., 2014a). Incubation with 0.1 mM of H2O2 exhibited similar changes in G′ despite the differences in magnitude. The magnitude of G′ at the peak was decreased by about 30 % compared with the control. Induction with H2O2 from 0.5 mM to 2.5 mM, the magnitudes of G′ at the peak were reduced by about 55 %, 63 % and 69 %, respectively. Meanwhile, it was worthy to see that shoulder peaks appeared around 55 to 65 °C. The minor transition could be assigned to the myosin tail-tail association due to MPs denaturation in mild oxidation conditions (Nagano et al., 1994; Xiong et al., 2010). The further addition of H2O2 (above 2.5 mM) greatly reduced the magnitude at the peak by around 78 %. The reduction in G′ of the oxidized MPs might cause alterations in the functional groups of MPs as shown in the physicochemical analysis. Moreover, the myosin content loss and MHC degradation also contributed to the decreasing of G′ (Fig. 1). The large protein aggregates formation hindered ordered interactions of reactive functional groups. It also had a negative effect on G′ (Zhou et al., 2014a).
During the cooling stage (Fig. 4B), the G′ of all samples gradually increased, indicating that continued cross-linking occurred among protein molecules. Apparently, in high salt content conditions, mild oxidation of MPs (H2O2 ≤ 1.0 mM) could greatly enhance gel stiffness. However, excessive oxidation (H2O2 ≥ 2.5 mM) could decrease gel stiffness. The maximum G′ values presented at 1.0 mM H2O2 addition. It had been reported that the increased G′ values was related to the formation of hydrogen bonds, which finally led to the increase of gel strength (Nagano et al., 1994). A negative correlation was observed between the G′ values and the particle size, which indicated that the mode of MPs aggregation was related to gel network formation. The changes in G′ values during cooling process were consistent with the results of gel texture and WHC (Fig. 3).
3.6 Microstructure Scanning electron microscopy (SEM) is usually used to depict gel morphologic properties (Horita et al., 2014). SEM micrographs of the control and H2O2-modified protein gels are shown in Fig. 5. These micrographs demonstrated significant effects of H2O2 treatments. As shown in Fig. 5, the control protein gel showed a spongy appearance with aggregates and uneven shapes. Addition of H2O2 ranged from 0.1 mM to 1.0 mM gradually improved the gel quality, turning the gel structure into a compact network with smooth surface. A highly dense structure was observed when the addition of H2O2 was 1.0 mM. It might be related to the fact that moderate protein oxidation caused cross-links of MPs, changing the aggregated gel structure into a well-oriented gel structure during heating treatment. However, the effect of further oxidation was inversed. Relatively high concentrations (≥ 2.5 M) of H2O2 increased uneven porous and surface. It was related to the cross-linked MPs, which were too large to retain ordered coalescence during the heating process of gel formation. These changes in structural features could explain the biphasic effect on gel strength and WHC as aforementioned (Fig. 3).

SEM images (magnification1: 1000) of MPs gels subjected to incubation of H2O2 at 0.5 M NaCl.
Results from this work indicated that MPs incubated with 0.5 M NaCl were more sensitive to •OH attacks due to expand sufficiently, resulting in variety of gel properties of MPs. Increases in the gel strength, WHC and the formation of dense, smooth, elastic gel network structure during heating process were observed under the conditions of 0.5 M NaCl combined with mild oxidation (1.0 mM H2O2). However, the excessive oxidation led to a significant reduction in gel qualities. These results will be favor of process control of meat production. This study could be helpful to understand the effect of oxidation on meat protein and improve process control of meat production.
Acknowledgements This work was supported by the National Key R&D Program of China (No. 2016YFD0401504); the National Natural Science Foundation of China (31671870); the Special Support Project of Guangdong Province for Science and Technology Innovative Young Talents (2014TQ01N538); and Pearl River S&T Nova Program of Guangzhou (201610010105).