2019 Volume 25 Issue 2 Pages 157-166
2019 Volume 25 Issue 2 Pages 157-166
Pulsed Electric Field processing is a non-thermal alternative to sucrose extraction from sugar beet. The aim of the study was to investigate the impact of pulsed electric field (PEF) treatment on the viscoelastic properties of sugar beet tissue. The electrical conductivity disintegration index was used for characterization of the PEF-induced damage of sugar beet tissue. Cell membrane disintegration increased with increasing field strength and pulse number. Stress-relaxation tests were also performed. The changes in viscoelastic properties were quantified as a result of applied field strength and pulse number and were correlated with cell disintegration index. The generalized Maxwell model was used to fit the measured stress–relaxation data. The rheological constants, elastic module (MPa), equilibrium elastic module (MPa) and relaxation time (s) were decreased significantly with increase of cell disintegration index. Scanning electron microscope images of the intact and PEF- treated samples showed increased deformation of cells and larger intercellular spaces for the PEF-treated samples.
The thermal treatment of sugar beet cossettes is traditionally used all over the world for sucrose extraction enhancement. In this process, sugar beets are cut into pieces (cossettes) and the sucrose is extracted by countercurrent water at high temperature (70–75 °C) during extracting times of 1–1.5 h (Van der Poel et al., 1998). Thermal extraction leads to pectin disintegration and release of undesirable substance in to the juice with the decrease of juice purity making it necessary to use costly and complicated juice purification (El Belghiti and Vorobiev, 2004; Loginova et al., 2011; Lopez et al., 2009(.
Pulsed electric field (PEF) processing is an efficient non-thermal food processing technique using short, high voltage pulses. These pulses induce poration of plant, animal and microbial cells and leading to cell disintegration. The moderate electric fields (MEF) treatment at electric field intensities (E), ranging within 0.5–1 kV/cm and treatment time of 10−4–10−2s leads to effective tissue damage. Depending on the electric field intensity and the number of pulses, induced changes at the plant tissue may be reversible or irreversible (Buckow et al., 2010; Grimi et al., 2010; Lebovka et al., 2007; Wang and Sastry, 2002).
MEF treatment is applied to improve the extraction process of different plant materials. Subjection of cellular plant material to external electric fields tends to creating pores in the cell membranes. This phenomenon is termed electroporation. Electroporation has been demonstrated to increase the permeability of the cell membrane. The biological cells have a natural potential difference across their membranes. If the electric field intensity exceeds the critical membrane potential (approximately 1V), dielectric breakdown of the cell membrane occurs that leads to reversible or irreversible pores formation. The advantage of PEF treatment is related to non-thermal damage of cellular membranes, which can be obtained without cell wall disintegration (Bazhal et al., 2003; Boussetta et al., 2013; Donsi et al., 2010; Knorr et al., 2001).
Recent studies have shown that application of MEF treatment can enhance the juice extraction and increase the mass transfer in the sugar beet (El-Belghiti and Vorobiev, 2004; Eshtiaghi and Knorr, 2002; Jemai and Vorobiev, 2003; Lebovka et al., 2007; Lopez et al., 2009; Loginova et al., 2011). Fincan et al. (2004), have also shown PEF (1 kV/cm) treatment as an effective method of permeabilization for the extraction of pigments from beetroot.
Pore formation and expansion leads to mechanical modification of the membrane. Conductivity and the viscoelastic properties have been studied to elucidate the PEF-induced changes at the structure of plant tissue. The electrical conductivity of a material refers to its ability to transport ionic species through the cell membrane pores and its changes related to permeabilization of the membrane (Bazhal et al., 2003; Fincan and Dejmek, 2002). After plasmolysis by different pre-treatment methods, tissue tends to be more soften and firmness is reduced (Krokida et al., 2001; Rojas et al., 2001). Previous researches have demonstrated textural changes in plant tissue after PEF-pretreatment (Bazhal et al., 2001; Fincan and Dejmek, 2003; Lebovka et al., 2004).
According to the aim of the application, the main challenge to utilization of electric field processing is the choice of an optimal PEF treatment mode, that is, electric field intensity (E), number of pulses (n) and PEF treatment duration (tPEF). The goal of the process optimization procedure is in obtaining quantifiable correlations between the processing protocol and the resulting plasmolysis or degree of damage. Nevertheless, the relationship between degree of cell disintegration and textural changes of sugar beet tissue under PEF treatment has not yet been studied. The objective of this investigation is to evaluate the influence of PEF-treatment on viscoelastic properties of sugar beet tissues and the relationship between cell disintegration index and textural changes in sugar beet tissue were also investigated and discussed.
Sample preparation Sugar beet was obtained from Shirin sugar factory (Mashhad, Iran). The beets were transported to Laboratory of Novel Food Technologies of Research Institute for Food Science and Technology (Mashhad, Iran) and subsequently cleaned and washed. Samples were sliced to a thickness of 1±0.1 cm and cut into cylindrical shapes of diameter 3.5 cm. All tests were performed in triplicate.
PEF equipment The laboratory pulsed electric field equipment was designed in Laboratory of Novel Food Technologies of Research Institute for Food Science and Technology (Mashhad, Iran). The schematic diagram of PEF equipment is illustrated in Fig. 1. The pulse frequency was 1 Hz. The PEF parameters used consisted of various field intensities (E=V/d, where E (kV cm−1) is electric field intensity, V (kV) is applied voltage and d (cm) is the distance between the electrodes) of 0.5, 0.75 and 1.5 kV cm−1, various numbers of exponential decay monopolar pulses (10, 20 and 30), 10 µs of pulse duration. The experiments were carried out in a plexiglass chamber between two stainless steel electrodes with 4 cm gap and electrode area of 50 cm2. The electrical resistance was 50 Ω in treatment chamber containing 30 mL tap water (EC = 0.5 mS). A 75 MHz high voltage probe was used to record the applied voltage. A high voltage generator charged the capacitor which was then discharged through the food material in tap water placed between parallel stainless steel electrodes. During PEF treatment, the temperature change was negligible.

Schematic diagram of PEF equipment.
Textural measurement All cylindrical pieces of sugar beet tissue before and after PEF were placed in the texture analyser to perform the stress relaxation test. The textural measurements were performed in a Texture Analyser (model TA-XT2, Stable Microsystems, UK). In the stress relaxation tests, the sample structure non-uniformity was compensated by sample preloading with a force of 0.1 N. The speed of piston displacement was 1 mm/s and the initial stress σ0 = 4F/πD2 ≈ 0.34 (MPa). Where F (N) is the compressional force, D, the diameter of the sugar beet disks. The corresponding strain was about 10%. All the stress relaxation curves were recorded with 0.1 s resolution. Each experiment was repeated at least five times.
Cell disintegration measurement The disintegration index (Z) indicates the degree of damaged (permeabilized) cells in the plant tissue and it has been estimated on the basis of the measurement of the electrical conductivity of intact and permeabilized tissue (Vorobiev and Lebovka, 2008). The electrical conductivity of samples were measured using conductivity meter model IDSC004 before and after PEF treatment when samples connected to a 12 V DC source. The disintegration index (Z) was calculated by the following equation:
 |
Where λ (S/m) is the measured electrical conductivity and the subscripts i and d refer to the conductivities of intact and completely damaged tissues, respectively. The value of λd was estimated from the measurements of electrical conductivity of a raw tissue that was frozen in a cold store (−20 °C) and then thawed. For the intact tissue, Z = 0 and for the completely disintegrated (Freeze-Thawed sample), Z = 1.
Mathematical model Stress relaxation data are useful in providing information about phenomena involving food processing. The stress relaxation test has been applied by several authors to study the viscoelastic behavior of fruit (Fincan and Dejmek, 2003; Lebovka et al., 2004; Lebovka et al., 2005; Peleg and Normand, 1983).
In stress relaxation tests, samples are subjected to an instantaneous deformation. This experiment can be done while in compression, extension, or shear. Deformation or strain is maintained constant throughout the test while the stress is determined as a function of time (Krokida et al., 1998; Steffe, 1996; Tabilo-Munizaga et al., 2005).
There are several mathematical models used to estimate the viscoelastic properties of fruit in stress relaxation tests, but the Maxwell model was used most frequently. The generalized Maxwell model is one of the most known models that is composed of several elements (a spring and a dashpot in series) in parallel that leads to better fittings.
The generalized Maxwell model, which is used to describe the relaxation modulus σ(t), consists of a spring and n Maxwell units connected in parallel as illustrated in Figure 2. Maxwell unit is a series arrangement of the elastic and viscous elements: an ideal spring in series with a dashpot.

Generalized discrete Maxwell model, σ, stress, MPa, E, the elastic module, MPa; η, specific viscosity, MPas.
In the Maxwell model with a constant strain (ε0), σ describes the compresion applied from σ0 for σ(t) after a time t given as follows (Eq. 2) (Nobile et al., 2007; Steffe, 1996):
 |
Where σ (t) (MPa) describes stress at any time; εo is a constant strain; E1 and Ee are the elastic modulus of the ideal elastic body and equilibrium moduli, respectively and τ is the relaxation time.
Most viscoelastic foods do not follow the Maxwell simplified model (Eq.2) and require more complex models to describe their stress relaxation curves.
The stress relaxation curves (stress versus time) can be interpreted by means of Equation (3), which provides the viscoelastic parameters of two component Maxwell model (Steffe, 1996).
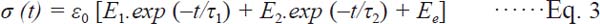 |
Where E1, E2 … are the elastic moduli of the ideal elastic body, Ee is the equilibrium elastic modulus and τ1, τ2 … are the relaxation times of each parallel element.
In this study, the possibility of using two component Maxwell model to estimate the stress relaxation behavior of sugar beet tissue subjected to PEF treatment is investigated and the effect of PEF treatment on the viscoelastic constants at a defined level of applied field strength and pulse number is also evaluated.
Scanning electron microscopy The microstructures of PEF treated and untreated sugar beet samples were investigated using scanning electron microscope (SEM). The images of slices of the sample tissue were obtained using an LEO 435-VP (Leo Electron Microscopy Ltd., England) operating at room temperature. For each experiment the 15 images from 3 different samples were analyzed and typical photographs were selected.
Data processing Experimental stress relaxation data vs. time were evaluated using Table Curve software (Table Curve 2D Windows, v4.07, SPSS Inc., AISN Software Inc., Chicago, USA)
Evolution of cell disintegration index Figure 3 shows values of disintegration index, Z, plotted against PEF treatment time (tPEF) at the different field strength. For intact cells, Z = 0; for total cell disintegration (freeze thawed tissue), Z = 1. The disintegration index increased with increasing PEF treatment time. The influence of the PEF treatment time on the cell disintegration can be described by electroporation theory. The increase of electric field strength, E, resulted in acceleration of electroporation and PEF-induced damage to the cell membrane. Increasing the electric field intensity from 0.5 to 0.75 led to a higher increase on the degree of cell disintegration. Increasing the electric field strength from 0.5 kV/cm to 0.75 kV/cm significantly (p < 0.05) affected values of Z. Increase of PEF treatment time from 2×10−4 to 3×10−4 at field strengths of 0.75 and 1.5 kV/cm, had no remarkable effect on the cell disintegration index (Fig. 3).

Cell disintegration index Z versus total pulse duration (tPEF) at different electric field strengths E.
These results agree with other investigation about the relationship between the membrane damage and the strength of the electric field and the number of pulses applied to the sample. Lebovka et al. (2001) have reported same result of threshold beyond which PEF has no further effect on cell disintegration.
Stress-relaxation test Figures 4–6 show the typical stress relaxation curves obtained for PEF treated sugar beet samples at field strength 0.5, 0.75 and 1.5 kV/cm and pulse number of 10, 20 and 30. As shown there is a sharp decrease in the stress after which the stress levels off to constant value. The solid line in the curves represents the Maxwell model.

Stress relaxation of sugar beet tissue subjected to different number of pulses (n) at a field strength (E) of 0.5 kV/cm and intact sample.

Stress relaxation of sugar beet tissue subjected to different number of pulses (n) at a field strength (E) of 0.75 kV/cm and intact sample.

Stress relaxation of sugar beet tissue subjected to different number of pulses (n) at a field strength (E) of 1.5 kV/cm and intact sample.
Maxwell model excellently fits the experimental data. The elastic moduli, E1, E2, and Ee, represent the elastic components in the Maxwell elements, and these are the measures of the elasticity of the material being tested (Kajuna et al., 1998). The relaxation time indirectly represents the viscous properties of the cell wall (Sakuria and Nevins, 1992).
The values of the Maxwell model elements as a function of different PEF conditions as well as the determination coefficient, R2, are listed in Table 1. As can be seen the Maxwell model fits very well with the experimental results for all PEF treated sample (0.987 ≤ R2 ≤ 0.998). It can be seen in the Table 1 PEF treatment had a decreasing effect on the first term of the two-term Maxwell model (E1, τ1).
| Electric field strength | Pulse number | Zp (Mean ± SD) | E1 (MPa) | E2 (MPa) | Ee (MPa) | τ1(s) | τ2(s) | R2 |
|---|---|---|---|---|---|---|---|---|
| Intact sample | 0 | 0.811 | 0.193 | 2.331 | 70.342 | 18.388 | 0.998 | |
| 0.5 kV/cm | 10 | 0.49±0.04 | 0.657 | 0.493 | 2.191 | 34.661 | 3.738 | 0.997 |
| 20 | 0.589±0.02 | 0.616 | 0.566 | 2.014 | 28.114 | 4.588 | 0.991 | |
| 30 | 0.651±0.05 | 0.589 | 0.862 | 1.745 | 27.284 | 3.520 | 0.993 | |
| 0.75 kV/cm | 10 | 0.792±0.05 | 0.556 | 0.972 | 1.724 | 23.461 | 4.793 | 0.996 |
| 20 | 0.894±0.05 | 0.488 | 1.338 | 1.549 | 23.926 | 2.501 | 0.987 | |
| 30 | 0.901±0.06 | 0.438 | 1.447 | 1.301 | 23.092 | 2.856 | 0.990 | |
| 1.5 kV/cm | 10 | 0.903±0.04 | 0.407 | 1.567 | 1.242 | 22.193 | 2.619 | 0.997 |
| 20 | 0.957±0.03 | 0.389 | 1.448 | 1.114 | 21.528 | 2.031 | 0.995 | |
| 30 | 0.961±0.04 | 0.360 | 1.61 | 0.979 | 20.436 | 1.501 | 0.993 | |
| Freeze thawed tissue | 1 | 0.346 | 1.308 | 0.748 | 18.212 | 1.102 | 0.991 | |
The modulus of elasticity is an indication of rigidity and stiffness of the material and not an indication of its degree of elasticity (Mohsenin and Mittal, 1977), this parameter can be used to quantify the textural changes of a material. The samples with higher averages of elastic modulus values are more rigid and hardest than the others (Peleg, 1987).
Textural tests have shown that first elastic moduli (E1) and equilibrium elastic moduli (Ee) of the PEF-treated samples are lower than those of the untreated control sample. The samples subjected to more intensive PEF treatment are more soften and in a less viscous behavior than the other ones, as their elasticity parameters had lower averages. A possible interpretation would be that PEF treatment destroys cell membranes, removes cellular turgor and causes a loss or decrease in the firmness of the tissue structure (Fincan and Dejmek, 2003; Lebovka et al., 2004; Lebovka et al., 2005).
The most pronounced change was the decrease in first and equilibrium elastic modulus, which already occurred at relatively low treatment levels and was paralleled by the decrease in the relaxation time. The relaxation behavior agrees with the result of Fincan and Dejmek (2003), who stated that the viscoelastic properties change in potato increased with the intensity of PEF treatment.
Relationship between the Maxwell model elements and cell disintegration index Cell disintegration index is a well-accepted indicator of PEF-induced damage to cellular tissue. Dependencies between the elastic moduli (E) for the first element, equilibrium elastic moduli and average relaxation time (τ) parameters, respectively versus cell disintegration index (Z) at different levels of PEF treatment are shown in Figures 7–9.

Relationship between elastic module and cell disintegration index

Relationship between equilibrium elastic module and cell disintegration index

Average relaxation time as a function of cell disintegration index
After the PEF treatment, the tissues lost a part of its initial strength, and the first and equilibrium elastic modulus decreased with increasing disintegration index (PEF treatment time). The tissue of fresh plant is composed of cells regularly distributed and intercellular spaces (Chassagne-Berces et al., 2009). The cells are bonded together by a connecting substance, and the tissue contains varying proportions of gaseous spaces, free liquids or even oils (Ruiz-Altisent, 1991). The viscoelastic properties of tissues related to turgor and properties of cell wall and middle lamella was demonstrated (Fincan and Dejmek, 2003; Scanlon et al., 1996; Warner et al., 2000).
The PEF treatment causes a non-thermal rupture of cellular membranes and decrease or loss of the turgor component of cells (Fincan and Dejmek, 2003; Lebovka et al., 2004). Loss of cell turgor, causing cell debonding and reduction of the mechanical modulus of vegetal tissues.
Destruction of the cell membranes as well as removal of the cellular turgor were defined as the main impact factors for the modification of texture and viscoelastic properties of plant tissue after PEF treatment. For all PEF experiments, the structure changes induced by external electric field seem to be less pronounced compared to freeze-thawed tissues. The maximal textural softening was observed for freeze-thawed tissues.
The relaxation time measured shows how fast the material dissipates stress after receiving a sudden deformation and as well as can be used to characterize the elastic and viscous parts in the behavior of a material. The relaxation time of the corresponding material in the Maxwell model can be obtained from the average of the several relaxation times of the model (Mohsenin, 1986). Figure 9 shows that the arithmetic averages of relaxation times were also affected by PEF treatment conditions. The value of τ decreases with increase of the conductivity index. A more intensive PEF treatment leads to larger pores in the cell membrane and thus leads to faster loss of cell liquid content and thereby faster relaxation (Fincan and Dejmek, 2003).
Lebovka et al. (2004, 2005) declared that the value of effective relaxation time for the apple and potato tissue decrease with increase of the electric field strength and the PEF treatment duration. They stated that the change in textural properties of the plant tissue refers to PEF-induced damage of cells. Materials that present a predominance of their viscous behavior (plastic) in detriment of its elastic character have smaller relaxation times, i.e., dissipate faster the applied stress.
Changes of microstructure Representative SEM micrographs of untreated and PEF treated sugar beet tissues (Figure 10) illustrate that there is a considerable difference in the microstructure of sugar beet tissues after PEF treatments with varied electric field strength and pulse number of 30. The observation of sugar beet tissue using scanning electron microscopy reveals that sugar beet cells in the untreated samples are intact, tightly pack with small intracellular spaces (Figure 10a).

Scanning electron microscopy (SEM) micrographs of intact sugar beet and treated sugar beet. (a) Intact sample; (b) PEF treated sample with 30 pulses and 0.5 kV/cm; (C) PEF treated sample with 30 pulses and 0.75 kV/ cm; (d) PEF treated sample with 30 pulses and 1.5 kV/cm; IS: intercellular space. CW: cell wall. CR: cell rupture.
During PEF treatment, SEM micrographs shows the alteration and perforation of cell wall, significant cellular deformation, small and irregular intercellular spaces and collapse of tissue (Figure 10b,c and d).
This alteration may be explained by the mechanism of electropermeabilization. When cells are permeabilized, channels are opened to allow diffusion into the tissue (Kulshrestha and Sastry, 2006). The cell wall permeabilization is correlated with the deterioration and destruction of the middle lamella (Siddiqui and Bangerth, 1996). These changes result in the loss of membrane integrity, plant firmness and quality of fruit and vegetable (Knorr 1999; Angersbach et al., 1999).
As reported in many studies, a change in electrical conductivity is a direct indication of cell membrane integrity loss (Knorr and Angersbach 1998; Lebovka et al., 2004; Arevalo et al., 2003). For solid cellular materials, such as plant and animal tissue, cell plasmolysis after PEF treatment may be explained by electroporation of the cellular membranes and reduction in the mechanical integrity of the cell wall (Rojas et al., 2001). As stated above, the decreased firmness resulted from reduced integrity in cell wall components and a consequence of losses in the hydrostatic pressure (turgor) within fruit cells.
Lebovka et al (2004) investigated the effect of pulsed electric field treatments on textural properties of carrots, potatoes, and apples; they stated that PEF treatment causes a nonthermal rupture of cellular membranes and decrease of the turgor components of cells. The higher the electric field strength applied the greater the potential to irreversibly alter the permeability of biological cells and thereby the appearance and properties of plant cells
The influence of PEF treatment on textural properties of sugar beet tissue has been studied. Acceptable degrees of cell disintegration were obtained for the different field strengths. The PEF-induced effects corresponded with increasing field strength and pulse number.
The stress–relaxation test for sugar beet tissue was performed. The results of the stress-relaxation test for sugar beet tissue showed that the experimental data fitted by the Maxwell model and can be used to describe the viscoelastic behavior of the samples. Our results clearly indicate that viscoelastic characteristics of sugar beet change with PEF treatment intensity. Viscoelastic parameters were obtained as a function of cell disintegration index. The pronounced decrease of the elastic modules with increase of cell disintegration index was observed that was paralleled by the decrease in the relaxation time. The relaxation times decreased with increasing cell disintegration suggesting that the samples treated at higher PEF treatment intensity dissipated the stress faster than the samples treated at lower PEF treatment intensity that causes by loss of turgor component of sugar beet texture. This suggest that electroplasmolysis affects not only plasmalemma membranes but also cell walls integrity of samples. The application of the PEF treatment results in softening effect of sugar beet tissue. SEM micrographs shows that pulsed electric field treatment causes noticeable cell deformation and structure collapse of texture.
Acknowledgements This study was approved by the Institutional Review Board of Research Institute of food science and technology (RIFST). The authors acknowledge Iran National Science Foundation (INSF) as well as Research Institute of food science and technology (RIFST) for financial support. Authors thank the Shirin sugar factory (Mashhad, Iran) for providing sugar beet.