2019 Volume 25 Issue 2 Pages 217-226
2019 Volume 25 Issue 2 Pages 217-226
Manual pipette methods are conventionally used to determine the viscosity (η) of gelatin solutions. The purpose of this study is to demonstrate the accuracy and precision of viscosity measurements of gelatin solutions using a rotational rheometer. Seven gelatin samples with different molecular weights were tested. The 2 × standard deviations of the obtained η were within ± 2.4% of the mean values in triplicate measurements, suggesting the evident precision of the viscosity measurements. Accuracy was demonstrated by the triplicate measurements of the standard liquids for a viscometer. The sample volume required by the rheometer was only 0.6 mL, whereas gelatin solutions of approximately 100 mL are usually required for pipette methods. We propose viscosity measurement using a rheometer as an alternative method to the pipette ones.
Gelatin is a kind of polypeptide that is thermally extracted from collagen-rich animal connective tissues, such as bones and dermis. The main component of gelatin is denatured collagen molecules (Liu et al., 2015). Its characteristic gelling properties (Djabourov et al., 1988) and processability to produce edible films (Sobral et al., 2001) and capsules (Gullapalli, 2010) are useful in food and pharmaceutical industries. Various forms of gelatin are mainly produced from gelatin solutions and dispersions. Therefore, the viscosities of gelatin solutions and dispersions are routinely specified in the industry.
Several standardized methods are established for testing various physicochemical properties of gelatin, including gel strength, melting and gelling temperatures, viscosity, pH, and ash content (Kobayashi, 2004). Methods for measuring the viscosity of gelatin are described in the Japanese Industrial Standard (JIS) K6503 method, the Gelatin Manufacturers Institute of America (GMIA) method, and the Gelatine Manufacturers of Europe (GME) method. All of them are manual measurements of fall velocities of gelatin solutions through glass pipettes.
Conventional pipette methods are simple and useful as routine tests in order to verify whether the viscosity of gelatin samples is within the error bound of specification. These methods, however, have at least three major limitations. Their accuracy and precision are not sufficient for detecting small differences in viscosity (<0.5 mPa s), and the required sample volume is large (>100 mL of 6.67 wt.% gelatin solutions). Moreover, the pipette methods cannot distinguish between Newtonian and non-Newtonian fluids. When a gelatin solution is a non-Newtonian fluid, the viscosity tested by the pipette method may not reflect viscous behaviors in practical uses.
Rotational rheometers have the potential to overcome the three aforementioned limitations. The detection sensitivity of torque (e.g., minimum 1 nN·m) enables accurate and precise viscosity measurements of gelatin solutions. The sample volume is equal to the gap volume between the upper and the bottom sensors, that is, <1 mL when a cone sensor having a diameter of 50 mm is used. The controllability of the rotational speed in a wide range (at shear rates from 10−3 to 103 s−1) allows the evaluation of non-Newtonian behaviors of polymer solutions, as described in JIS K7117-2 method. Rotational rheometers have been frequently used for linear viscoelastic measurements of gelatin solutions under oscillation mode to evaluate the sol-gel transition properties (Djabourov et al., 1988; Godard et al., 1978; Lai and Lii, 1997; Normand et al., 2000; Osorio et al., 2007 Tomczyńska-Mleko et al., 2014). In contrast, rotational viscosity is just supplemental data for the sol-gel transition properties (Joly-Duhamel et al., 2002; Karaman et al., 2016). The accuracy and precision of the rotational viscosity measurements of gelatin solutions have not been reported so far; thus, it is still unclear whether rotational rheometers are useful and have no limitation for quality control of gelatin.
The purpose of this study is to demonstrate the accuracy and precision of viscosity measurements of gelatin solutions using a rotational rheometer. The inherent accuracy and precision of the rheometer were investigated using low viscous standard mineral oils. A custom-made sensor hood to humidify the sample solutions was developed in order to ensure accurate and precise viscosity measurements. The precision of gelatin solutions was quantified in triplicate measurements.
Materials Commercial-grade gelatin samples I–VII (Table 1) were all provided by Nitta Gelatin (Osaka, Japan). Other chemicals were all purchased and used without further purification, including pepsin-digested and purified collagen from porcine skin in dilute HCl (pH 3) (Collagen BM; 0.53% solution of mainly type I collagen, Nitta Gelatin), standard liquids for calibrating viscometers (JS2.5, JS5, JS50, and JS100; Nippon Grease, Yokohama, Japan), silicone oil (KF-96L-1CS; Shin-Etsu Chemical, Tokyo, Japan), disodium hydrogen phosphate (Na2HPO4) (Wako Pure Chemical Industries, Osaka, Japan), sodium dihydrogen phosphate (NaH2PO4) (Wako Pure Chemical Industries), and guanidine hydrochloride (GDH) (Wako Pure Chemical Industries).
| Namea | Sources | pIb | Sedimentation coefficientc [S] | ηd [mPa s] | ||
|---|---|---|---|---|---|---|
| Number average | Weight average | Mean ± 2SD | % 2SDe | |||
| I | Porcine skin | 5 | 1.08 | 1.23 | 2.59 ± 0.06 | 2.4 |
| II | Porcine skin | 5 | 2.05 | 2.51 | 8.34 ± 0.15 | 1.8 |
| III | Porcine skin | 9 | 1.61 | 1.83 | 6.99 ± 0.04 | 0.5 |
| IV | Porcine skin | 9 | 1.78 | 2.10 | 7.81 ± 0.08 | 1.0 |
| V | Bovine bone | 5 | 1.95 | 2.74 | 8.50 ± 0.18 | 2.1 |
| VI | Bovine bone | 5 | 2.24 | 3.24 | 10.45 ± 0.09 | 0.8 |
| VII | Bovine bone | 5 | 2.47 | 3.81 | 11.81 ± 0.07 | 0.6 |
 of 57 s−1 (n = 3).
of 57 s−1 (n = 3).Rheometer An MCR302 rheometer (Anton Paar, Innsbruck, Austria) with the upper sensor CP50/1 (diameter = 50 mm, cone angle = 1°) was employed to measure the viscosities of the standard liquids and gelatin solutions in both oscillation and rotation modes. The rheometer was equipped with a Peltier-controlled hood as well as a Peltier-controlled bottom to switch sample temperatures rapidly.
Environmental settings of the sensor of the rheometer Three environmental settings of the sensor were tested in order to evaluate the effects of drying prevention on viscosity measurements. The normal setting of the rheometer is comprised of the upper sensor and the horizontal bottom, which are both covered by the originally equipped Peltier-controlled hood. The normal setting, without using the Peltier-controlled hood, was employed to accelerate air drying of the sample solutions (designated as “Setting-A”). One setting for drying prevention employed a custom-made sensor hood (Fig. 1) (designated as “Setting-B”). The custom-made hood was comprised of a ring-shaped outer fence (diameter = 84 mm, height = 14 mm) surrounding the sensors, a ring-shaped inner fence (diameter = 65 mm, height = 10.5 mm) having 90 holes (diameter = 2.5 mm, porosity = 21%), and a couple of semicircular lids that were placed on the outer fence. The horizontal bottom (diameter = 50 mm) was incorporated into the custom-made hood. Felt paper with a thickness of 2 mm was not only inserted into the space between the outer and the inner fences but also placed on the lids. Water was absorbed by the felt paper to create a humid environment. The custom-made hood was covered by the Peltier-controlled hood during the rheometer's operation. The other setting for drying prevention involved the coverage of the silicone oil (designated as “Setting-C”). The custom-made hood was set without the lids, in which the silicone oil was placed to achieve a level of the oil surface slightly above the edge of the upper sensor. The Peltier-controlled hood was used during the operations.

Custom-made sensor hood for the rheometer to create a humid environment. (A) A schematic drawing of the sensor and custom-made hood. The upper cone sensor CP50/1 (diameter = 50 mm, cone angle = 1°) (1) was used in all measurements. The horizontal bottom sensor (2) was incorporated in the custom-made hood. The custom-made hood was comprised of a ring-shaped outer fence surrounding the sensors (3), a ring-shaped inner fence with 90 holes (4), and a couple of semicircular lids that were placed on the outer fence (5). Felt paper (6) was placed on the lids and inserted into the space between the outer and the inner fences. (B) The appearance of the custom-made hood without the lids. (C) The appearance of the lids.
Assessments of the accuracy and precision of viscosity measurements The rotational viscosities (η) of the standard liquids—JS2.5, JS10, and JS50—were measured using the rheometer in the rotation mode at the normal setting at 40 °C. An aliquot of each liquid was applied to the rheometer. Then, the shear rate ( ) was increased stepwise from 1 to 1,000 s−1. The delay and integration time of each step to acquire η were determined in the manner of logarithmic division, that is, 200 s at 1 s−1 and 5 s at 1,000 s−1. Measurements of η were performed for triplicate samples. The value of η obtained at each
) was increased stepwise from 1 to 1,000 s−1. The delay and integration time of each step to acquire η were determined in the manner of logarithmic division, that is, 200 s at 1 s−1 and 5 s at 1,000 s−1. Measurements of η were performed for triplicate samples. The value of η obtained at each  was averaged and expressed as a percentage of the corresponding certificate value [mean ± 2 × standard deviation (2SD)]. η was considered to be sufficiently accurate and precise when the deviation bounds of η were within ±5% of the corresponding certificate value.
was averaged and expressed as a percentage of the corresponding certificate value [mean ± 2 × standard deviation (2SD)]. η was considered to be sufficiently accurate and precise when the deviation bounds of η were within ±5% of the corresponding certificate value.
Effects of drying prevention on viscosity measurements of gelatin solutions The viscosity of the gelatin-VII solution was measured in the oscillation mode under the three settings (Setting-A, Setting-B, and Setting-C) in order to investigate the effects of drying prevention of the solution. Gelatin-VII granules were soaked in deionized water in 50 mL biological tubes to achieve a concentration of 6.67 w/w% and were then inverted and stirred at room temperature for 120 min, before heating in a water bath at 60 °C for 30 min to achieve complete dissolution. An aliquot of the gelatin solution (approximately 0.6 mL, preheated at 60 °C) was poured onto the bottom sensor at 40 °C, and then the three settings were applied. After rotation at  of 60 s−1 for 1 min to erase the flow history, the measurement mode was changed to oscillation mode (frequency = 0.5 Hz, shear stress = 0.5 Pa). The dynamic viscoelastic measurement was continued for 60 min to monitor the change of complex viscosity (η*).
of 60 s−1 for 1 min to erase the flow history, the measurement mode was changed to oscillation mode (frequency = 0.5 Hz, shear stress = 0.5 Pa). The dynamic viscoelastic measurement was continued for 60 min to monitor the change of complex viscosity (η*).
Acquisition of viscosity curves of gelatin solutions The η values of the 6.67 w/w% solutions of the gelatin samples listed in Table 1 were measured in the rotation mode under Setting-B in a wide range of  . The gelatin solutions were prepared as described above. An aliquot of each individual gelatin solution was applied to the rheometer at 40 °C, and then preshearing was conducted similarly to the above experiments for drying prevention. Then,
. The gelatin solutions were prepared as described above. An aliquot of each individual gelatin solution was applied to the rheometer at 40 °C, and then preshearing was conducted similarly to the above experiments for drying prevention. Then,  was increased stepwise from 3 to 3,000 s−1. The delay and integration time of each step to acquire η were determined in the manner of logarithmic division, that is, 180 s at 1 s−1 and 3 s at 3,000 s−1. Measurements were performed for triplicate samples. The value of η obtained at
was increased stepwise from 3 to 3,000 s−1. The delay and integration time of each step to acquire η were determined in the manner of logarithmic division, that is, 180 s at 1 s−1 and 3 s at 3,000 s−1. Measurements were performed for triplicate samples. The value of η obtained at  of 57 s−1 was averaged. The percentages of 2SD to mean η were calculated to indicate the precision of the data.
of 57 s−1 was averaged. The percentages of 2SD to mean η were calculated to indicate the precision of the data.
One-shot viscosity measurements including multiple temperature steps The η value of 6.67 w/w% gelatin-VII solution was measured in the rotation mode under Setting-B. The temperature was increased in a stepwise manner. An aliquot of preheated gelatin solutions was applied to the rheometer at 30 °C. Rotation at  of 50 s−1 was initiated, and the temperature was maintained at 30 °C for 15 min. The temperature steps were as follows: 30 °C for 15 min, 40 °C for 15 min, and 50 °C for 15 min. The transits of temperature were arbitrarily carried out.
of 50 s−1 was initiated, and the temperature was maintained at 30 °C for 15 min. The temperature steps were as follows: 30 °C for 15 min, 40 °C for 15 min, and 50 °C for 15 min. The transits of temperature were arbitrarily carried out.
Evaluations of the molecular weights of gelatin samples using analytical ultracentrifugation The sedimentation coefficients of the gelatin samples were evaluated by sedimentation velocity experiments using an analytical ultracentrifugation apparatus (ProteomeLab XL-I; Beckman Coulter, San Diego, CA, USA). The granular gelatin samples that are listed in Table 1 were dissolved in 4 M GDH (pH = 7), buffered using 50 mM Na2HPO4/NaH2PO4. The 1.0 w/v% gelatin solutions with an approximate volume of 500 µL were dialyzed using Micro Float-A-Lyzer (MWCO 500–1000 Da; Spectrum Labs, Rancho Dominguez, CA, USA) against the phosphate-buffered GDH solutions (an approximate volume of 100 mL). The outer solutions were exchanged twice. The inner solutions were diluted after dialysis using the final outer solutions to achieve an absorbance of 0.6–0.8 at 230 nm. In addition to the gelatin solutions, the collagen in the phosphate-buffered 4 M GDH was prepared and used as a reference to the gelatin samples. The dialyzed gelatin solutions and the outer solutions used as references were loaded into the cells. Ultracentrifugation at 60,000 rpm (250,000 × g) was run at a chamber temperature of 20.0 °C (±0.1 °C). Single scan absorbance data at 230 nm were acquired at a scan interval of 3 min and accumulated to achieve a total scan number greater than 100. Experimental sedimentation profiles of the gelatin samples were analyzed with the program SEDFIT (sedfitsedphat.nibib.nih.gov) using the sedimentation coefficient distribution c(s) model (Schuck, 2000), with a resolution of 300 between 0 and 15 S (S is a Svedverg unit that is defined as 10−13 × sedimentation coefficient; a larger S reflects a larger molecular weight). In the c(s) analysis, the partial-specific volume was set to 0.73 mL g−1. The buffer density and viscosity were calculated by SEDNTERP (sednterp.unh.edu) to be 1.288 g mL−1 and 0.01103 cp, respectively, which were used for the c(s) analysis. The quality of the data fit was evaluated using a root mean square deviation of less than 0.01. The obtained c(s) (unit: absorbance S−1) was normalized to achieve the integrated c(s) of 1.0 in the sedimentation coefficient range of 0–15 S, where abruptly high c(s) in the sedimentation coefficients <0.1 S and 14.8–15 S was deleted as noises of the analysis. The number-averaged (NAS) and weight-averaged sedimentation coefficients (WAS) were calculated to indicate the molecular weights (MWs) of the gelatin samples. Components with sedimentation coefficients greater than or equal to 3.2 S were defined as high-MW (HMW) components.
Accuracy and precision of viscosity measurements using a rheometer Figures 2A–2C show the viscosity curves and the torque data of the standard liquids—JS2.5, JS10, and JS50—obtained at 40 °C. The three liquids were chosen because their η values are similar to those of the 6.67 wt.% solutions of commercially available gelatins. The measurement temperature was equal to that of the conventional method [6]. The torque increased proportionally by increasing  , suggesting a Newtonian behavior that is typical for mineral oils (i.e., η is constant irrespective of
, suggesting a Newtonian behavior that is typical for mineral oils (i.e., η is constant irrespective of  ). The obtained η (mean ± 2SD) was expressed as a percentage of the corresponding certificate value (Figs. 2D–2F). In JS50, the deviation bounds of η were within ±5% of the certificate values at all the examined
). The obtained η (mean ± 2SD) was expressed as a percentage of the corresponding certificate value (Figs. 2D–2F). In JS50, the deviation bounds of η were within ±5% of the certificate values at all the examined  (Fig. 2F). This result indicated that the viscosity that was measured using the rheometer was accurate and precise when the torques generated by the sample solutions were sufficiently high (>0.59 µN·m). The deviations of η in JS10 were also within ±5% of the corresponding certificate values at all the examined
(Fig. 2F). This result indicated that the viscosity that was measured using the rheometer was accurate and precise when the torques generated by the sample solutions were sufficiently high (>0.59 µN·m). The deviations of η in JS10 were also within ±5% of the corresponding certificate values at all the examined  at 30 °C, except for the data at
at 30 °C, except for the data at  of 1.9 s− (at a torque of 0.28 µN·m) (Figs. 2B and 2E). The deviation bounds of η in JS2.5 were within ±5% of the corresponding certificate values at
of 1.9 s− (at a torque of 0.28 µN·m) (Figs. 2B and 2E). The deviation bounds of η in JS2.5 were within ±5% of the corresponding certificate values at  ≥ 10 s−1 (Fig. 2D). The data were obtained at torques greater than or equal to 0.44 µN·m (Fig. 2A). In contrast, the data obtained at low
≥ 10 s−1 (Fig. 2D). The data were obtained at torques greater than or equal to 0.44 µN·m (Fig. 2A). In contrast, the data obtained at low  (≤7.2 s−1) showed large deviation bounds over ±5% of the certificate value of JS2.5. The enlarged deviations reflected the fluctuation of the viscosity curve (Fig. 2A), in which the torques were only 0.04–0.32 µN·m.
(≤7.2 s−1) showed large deviation bounds over ±5% of the certificate value of JS2.5. The enlarged deviations reflected the fluctuation of the viscosity curve (Fig. 2A), in which the torques were only 0.04–0.32 µN·m.

Results of viscosity measurements of standard mineral oils by the rheometer. The η values (●) and torques of JS2.5 (A), JS10 (B), and JS50 (C) at 40 ° (○) as a function of  . Measurements of η were performed for triplicate samples. The η values obtained at each
. Measurements of η were performed for triplicate samples. The η values obtained at each  were averaged and expressed as a percentage of the corresponding certificate value (mean ± 2SD). (D), (E), and (F) are the converted data from (A), (B), and (C), respectively.
were averaged and expressed as a percentage of the corresponding certificate value (mean ± 2SD). (D), (E), and (F) are the converted data from (A), (B), and (C), respectively.
Effects of drying prevention on viscosity measurements with a rheometer The time-dependent changes of η* were compared among the three environmental settings of the sensor. As shown in Fig. 3, the largest increase in η* (an increase of 33% in 10 min) was observed in Setting-C using the silicone oil coverage. When the marginal region of the specimen was exposed to the atmosphere under Setting-A, η* increased by 9.6% in 20 min. In contrast, great suppression in the increase of η* was observed in Setting-B, and the rate of increase was only 2.6% in 60 min.

Time-dependent changes of viscosity (η*) of gelatin solutions obtained by the rheometer in the oscillation mode under three environmental settings. (A) The narrow solid line, broken line, and bold solid line indicate the data obtained by Setting-A, Setting-B, and Setting-C, respectively. η* was measured in the oscillation mode using the rheometer at 40 °C. The 6.67 wt.% solution of gelatin-VII was used as a sample solution. (B) η* was normalized to the percentages of the initial values. Open circles, open squares, and closed circles indicate the data obtained by Setting-A, Setting-B, and Setting-C, respectively
η of Gelatin Solutions Figure 4 shows the viscosity curves of the gelatin solutions. The values of η were different among the samples. Gelatin samples with pI = 5 from porcine skin (gelatin-I and gelatin-II) and bovine bone (gelatin-V, gelatin-VI, and gelatin-VII) exhibited typical Newtonian behaviors, in which the values of η were invariable with  (Figs. 4A and 4C). In contrast, a non-Newtonian behavior was observed for gelatin samples with pI = 9 from porcine skin (gelatin-III and gelatin-IV). The values of η were intermediate among the samples (Fig. 4b).
(Figs. 4A and 4C). In contrast, a non-Newtonian behavior was observed for gelatin samples with pI = 9 from porcine skin (gelatin-III and gelatin-IV). The values of η were intermediate among the samples (Fig. 4b).

Viscosity curves of gelatin samples I–VII obtained at 40 °C using the rheometer in the rotation mode. The 6.67 wt.% solution were used for the tests. (A) Gelatin with pI = 5 obtained from porcine skin. (B) Gelatin with pI = 9 obtained from porcine skin. (C) Gelatin with pI = 5 obtained from bovine bone. Roman numerals in the figure indicate the names of the gelatin samples.
The η values of the gelatin solutions obtained at  of 57 s−1 are shown in Table 1. These values were 2.59–11.81 mPa s. The percentages of 2SD to mean η were in the range of 0.5–2.4%, indicating the precision of the measurements.
of 57 s−1 are shown in Table 1. These values were 2.59–11.81 mPa s. The percentages of 2SD to mean η were in the range of 0.5–2.4%, indicating the precision of the measurements.
One-shot viscosity measurements including multiple temperature steps Figure 5 shows the results of viscosity measurements of gelatin-VI at multiple temperature steps of 30, 40, and 50 °C without changing the sample solution. At the first temperature level (30 °C), η gradually increased in a time-dependent manner probably because of the partial renaturation of gelatin molecules to triple-helical collagen molecules. After the temperature started to increase from 30 °C, the sensor temperature was stabilized at the second temperature level (40 ± 0.1 °C) in 4 min. At the same time, η became stabilized at 11.9 ± 0.1 mPa s. The third temperature level of 50 ± 0.1 °C and stabilized η at 9.5 ± 0.1 mPa s were achieved in 2 min after the temperature started to increase from 40 °C.

Results of viscosity measurements of 6.67 wt.% gelatin-VII solution at multiple temperature steps. The solid and broken lines indicate η and temperature, respectively.
Estimations of MW distributions of gelatin samples The c(s) distributions of the gelatin samples were evaluated using analytical ultracentrifugation to estimate their MW distributions (Fig. 6). The peaks in the c(s) distributions could be assigned from the results of the purified collagen samples (Figs. 6A and 6B): α-, β-, and γ-chains at sedimentation coefficients of 1.6, 2.2, and 2.7 S, respectively. The α-chain contributed to the largest peak. Compared with the c(s) distribution of the purified collagen sample, evident decreases in the sedimentation coefficient were observed in the c(s) distribution of gelatin-I (Figs. 6C and 6D). The c(s) distribution of gelatin-VII showed that this gelatin sample mainly consisted of collagen chains and contained other minor components having sedimentation coefficients lower than that of α-chain (<1 S) and higher than that of γ-chain (>3 S). Gelatin-VII, thus, had a broad MW distribution compared with the purified collagen samples that were denatured with GDH. The HMW contents monotonously increased as NAS and MAS increased (Fig. 6G).

Evaluations of sedimentation coefficients of gelatin samples by sedimentation velocity experiments using an analytical ultracentrifuge. (A), (B), and (C) are c(s) distributions of purified collagen sample, gelatin-I, and gelatin-VII, respectively. (D), (E), and (F) are magnifications of (A), (B), and (C), respectively. NAS (closed circles) and WAS coefficients (open circles) were plotted against the contents of HMW components, which were defined as components having sedimentation coefficients higher than that of the γ-chain (G).
Relationship between η and average sedimentation coefficients The η values of all the gelatin samples were plotted against NAS (Fig. 7A). The plot showed a monotonous increase in η with the increase in NAS. An almost linear correlation yielded formula (1) by least-squares analysis (R2 = 0.981):
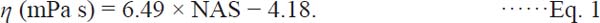 |

Plots of η of all the gelatin samples against NAS (A) and WAS (B). NAS: number-average sedimentation coefficients obtained by sedimentation velocity experiments using an analytical ultracentrifuge. WAS: weight-average sedimentation coefficients obtained by the same experiments. The equation and R2 in (A) are the results of the least-squares analysis.
The plot of η against WAS also showed a monotonous increase in η, although the correlation was not linear (Fig. 7B).
In the present study, we revealed the potential of a commercially available rotational rheometer for determining the viscosity of low viscous gelatin solutions. Rotational rheometers have been used for evaluating viscous properties of globular protein (Sharma et al., 2011), antibody (Kanai et al., 2007), and polysaccharide solutions (Tuinier et al., 1999) of which the viscosities were as low as < 10 mPa s. These studies suggested that viscosity measurements of low viscous (viscosity 1–20 mPa s) gelatin solutions are useful for the quality control of gelatin. However, quantified accuracy and precision of viscosity measurements of gelatin solutions have not been reported so far. To address this issue, we conducted a series of investigations to evaluate accuracy and precision of viscosity measurements of gelatin solutions with a rotational rheometer by comparing the data to those of certified viscosity standard liquid.
Compared with the conventional pipette methods, the outstanding advantage of the method using a rheometer is the accuracy and precision of the obtained η. This precision was evident from the result that the 2SD of η was within ±2.4% of the mean values in triplicate measurements (Table 1). The accuracy was demonstrated by the triplicate measurements of the standard liquids with η values similar to those of the gelatin solutions (Fig. 2): the 2SD of η was within ±5% of the certificate values. The JIS K7117-2 method defines that the allowance of η measured by rheometers is ±5%. Therefore, a single test could be enough to determine η of a 6.67 wt.% gelatin solution. In contrast, the pipette methods employ stopwatches to measure fall velocities of gelatin solutions manually, inevitably reducing the accuracy and precision as well as resulting in potential errors depending on the operators.
The accuracy and precision of the obtained η by the rheometer are attributed to the excellent torque sensitivity of the rheometer (1 nN·m, catalog data of the MCR302 rheometer). In the tests for the standard liquid JS2.5, reliable data could be obtained at very low torques (≥0.44 µN·m). η was determined to be 1.36 ± 0.04 mPa s at  of 51 s−1 (Figs. 2A and 2D). This sensitivity could be sufficient for most commercially available gelatins, because most degraded gelatin-I (Figs. 6C and 6D) still showed η of 2.59 ± 0.06 mPa s.
of 51 s−1 (Figs. 2A and 2D). This sensitivity could be sufficient for most commercially available gelatins, because most degraded gelatin-I (Figs. 6C and 6D) still showed η of 2.59 ± 0.06 mPa s.
In order to ensure the accuracy and precision of the viscosity measurements, the sensor environment of the rheometer needs to be humidified, especially in tests whose duration is over 10 min (Fig. 3). Gelatin is an adhesive material that is used as “animal glue” (Hull and Bangert, 1952; Schellmann, 2007), immediately forming adhesive membranes on the solution surface by air drying. The concentration of the tested gelatin solutions was 6.67 wt.%, which is common for JIS, GMIA, and GME test methods. The high concentration accelerated the formation of membranes on the surface of gelatin solutions, thereby increasing torques during viscosity measurements without the humidification of the sensor environment (Fig. 3). This problem could not be encountered in measurements of protein and polysaccharide solutions because these biopolymers show almost no adhesive properties. In addition, the measurements can be performed at ambient temperatures because the solutions do not form gels at the temperatures. In the present study, we developed a sensor hood to humidify the sensor environment (Fig. 1). This hood contained water-absorbing felt paper inside of it and on the back of the lids, which ejected water vapor under warmed conditions. The lids maintained the humid environment. Reliable viscosity measurements could be performed for at least 60 min (Fig. 3). When the sensors were covered with silicone oil to suppress the drying of gelatin solutions, η of the gelatin solution increased immediately and greatly in a time-dependent manner (Fig. 3). The reason of this fault is unknown, but interactions between water and the low MW silicone oil (kinematic viscosity is only 1 cSt) should be considered.
The much smaller sample volume for the rheometer (0.6 mL) compared with that of the pipette method (approximately 100 mL) is the second advantage. Recent developments in biomedical science, including regenerative medicine and tissue engineering, have encouraged gelatin manufacturers to develop biological-grade gelatins. Actually, gelatin is frequently used in regenerative medicine and tissue engineering (Echave et al., 2017). Biological-grade gelatins must be sterilized to allow their in vitro and in vivo uses. Clinical applications require trace amounts of endotoxins in gelatin samples. The manufacturing processes for sterilization and endotoxin reduction extremely increase the cost of gelatins. Thus, such a small sample volume required by rheometers is useful, especially for biological-grade gelatins.
The third advantage of a rheometer concerns the non-Newtonian behaviors of gelatin solutions. It has been well accepted that gelatin solutions are Newtonian liquids. Wulansari et al. (1998) demonstrated the Newtonian behaviors of gelatin solutions, while the viscosities were measured at a high temperature (50 °C). However, the solution of gelatin-III showed a non-Newtonian behavior at 40 °C (Fig. 4B), at which the physical properties of gelatin solutions can be reliably obtained because the molecular aggregations and degradation are both minimized (Boedtker and Doty, 1954). Although the data of the molecular weight distribution are still company's own data and have not yet been shown in published literature, high-molecular-weight gelatins with improved gelation properties have recently been developed and used for biomedical applications (Matsui and Tabata, 2012; Takagi et al., 2018). High-molecular-weight components tend to generate molecular entanglements, which cause shear thinning at high  . Thus, the poor dissolution of gelatin could be detected from viscosity curves in a wide range of
. Thus, the poor dissolution of gelatin could be detected from viscosity curves in a wide range of  obtained by a rotational viscosity test. This is an advantage of a rotational rheometer over the pipette methods, which cannot detect non-Newtonian behaviors.
obtained by a rotational viscosity test. This is an advantage of a rotational rheometer over the pipette methods, which cannot detect non-Newtonian behaviors.
The ability of a rheometer to switch sample temperatures rapidly enables us to obtain η at multiple temperatures in a single test (Fig. 5). This is an additional advantage of rheometers. Gelatin solutions are conventionally tested at 40 °C. However, tests at different temperatures are frequently required by manufacturers in order to estimate viscous properties in practical uses. In addition, the commercial software for rheometers allows creating various temperature profiles in accordance with the purposes of the tests. The rheometer has the potential to perform both viscosity measurements and determinations of gelling and melting temperatures continuously without changing sample solutions. Although the viscosity data at the constant  (50 s−) was immediately obtained at 50 °C, it should be noted that the measurement time may be reduced at the temperature or more because of accelerated drying of sample solutions, even if the custom-made sensor hood is used.
(50 s−) was immediately obtained at 50 °C, it should be noted that the measurement time may be reduced at the temperature or more because of accelerated drying of sample solutions, even if the custom-made sensor hood is used.
The η value that was obtained using the rheometer correlated well with NAS (Fig. 7A), which corresponded to number-average MW. It is difficult to convert c(s) distributions obtained by sedimentation velocity experiments using an analytical ultracentrifuge to MW correctly. The results in Fig. 7A, however, suggested that the MW of gelatin was determined by viscosity measurements using a rheometer. In our viscosity tests, seven gelatin samples with extremely different average MW and HMW contents (Fig. 6) were used. The collagen chains of gelatin-I were almost completely degraded, whereas the major components of gelatin-VII with the highest NAS and WAS were collagen chains. Viscosity measurements using a rheometer could be reliable irrespective of the MWs of gelatin samples.
In conclusion, we demonstrated the accuracy and precision of viscosity measurements of gelatin solutions using a rotational rheometer. The very small sample volumes and the ability to distinguish Newtonian from non-Newtonian behaviors are advantages over the conventional pipette methods. Moreover, rheometers could perform viscosity measurements at multiple temperature levels in a single test without changing the sample solutions. Measurements could be reliable irrespective of the MWs of gelatin samples. Hence, we propose viscosity measurements using a rheometer as an alternative method to the pipette methods.
Acknowledgements This work was supported in part by Adaptable and Seamless Technology Transfer Program through Target-driven R&D, Japan Science and Technology Agency Grant Number AS2815117U.