2019 Volume 25 Issue 3 Pages 467-477
2019 Volume 25 Issue 3 Pages 467-477
We have reported that the ingestion of a drink including winter savory extract (WSE) has an effect on core body temperature (CBT) and body surface temperature (BST) in people who experience cold sensitivity. In this study, to elucidate how the swallowing process contributes to changes in CBT and BST, experiments comparing the effects of WSE-drink and WSE-capsule were performed. We showed that a small amount of WSE-capsule did not affect CBT and BST, but WSE-drink (containing an equal amount of active principles in the WSE-capsule) induced significant changes in peripheral BST without changes in CBT. Moreover, WSE-drink with a higher concentration but equal amount of active principles induced significant changes in both CBT and BST. These results suggest that neurostimulation in the swallowing process after drinking WSE might greatly contribute to heat production and heat transfer (which is caused by peripheral vasodilation) independently of each other.
Terasawa (1987) reported that cold sensitivity (the feeling of cold; hie-sho in Japanese) is defined as a chilly sensation in a particular part of the body such as the lower back or, upper and lower limbs. Cold sensitivity is caused by the vasoconstriction of peripheral vessels, followed by reduction of skin blood flow (Nagashima et al., 2002; Ushiroyama et al., 2005).
Winter savory (Satureja montana L.), a herb of the Lamiaceae family, is known not only as a food condiment and herbal tea ingredient, but also as a food ingredient with physiological activity (Başer, 1995; Momtaz and Abdollahi, 2008). Herbal tea prepared from dried leaves of winter savory has a characteristic flavor which consists of herbal notes and a mild, pepper-like pungency (Burdock, 2010).
We have reported that winter savory extract (WSE) induced increases in core body temperature (CBT), measured at the tympanic membrane, and body surface temperatures (BSTs), measured at the wrist, finger, ankle, toe, forehead and neck, compared with those induced by the ingestion of a control in individuals who experience cold sensitivity after ingestion in capsule form (Masuda et al., 2011 and 2016a).
The ingestion form of food or medicine is considered to be an important factor for highly physiological action (Sakai, 2000; Shigenobu and Ishii, 2007). The number of sites of action expressing physiological activity (oral cavity, throat, esophagus, stomach and intestines) after the ingestion of a drink containing WSE (WSE-drink) is more than that (stomach and intestines) after the ingestion of a capsule containing WSE (WSE-capsule). In a previous study, we reported that WSE-drink induced increases in CBT and BST, compared to a control, in people who experience cold sensitivity (Masuda et al., 2017). Comparing the ingestion of WSE-drink and that of WSE-capsule, we suggested that neurostimulation in the oral cavity and throat by WSE-drink might be involved in changes in body temperature. However, a part of the clinical trials was carried out using an open-label design, which may lead to subjective effects, such as a placebo effect or a stress reaction; therefore, a clinical trial using double-blind conditions should be performed.
As for the active principles in WSE, it was proposed that carvacrol (the main volatile component in WSE) and thymol (the second major volatile component in WSE) might greatly contribute to the increase in CBT and the inhibition of the decrease in BST, respectively (Masuda et al., 2013 and 2016b). Their effect on body temperature might be induced by the activation of transient receptor potential (TRP) channels (which are non-selective cation channels present in perivascular sensory nerves and show a variety of physiological functions (Holzer, 2011)) in the gastrointestinal tract (Masuda et al., 2016a). However, after the ingestion of WSE-drink, how the swallowing process (oral cavity, throat and esophagus) affects CBT and BST remains unclear. As for carvacrol and thymol, it has been reported that lingual warmth was induced by their activation of TRP channels in the oral cavity (Xu et al., 2006; Klein et al., 2013 and 2014); however, the effects on CBT and BST have not been reported. Therefore, clarifying the detailed action mechanism leading to the changes in body temperature induced by the swallowing process after the ingestion of WSE-drink is considered to be useful to understand how the swallowing process contributes to CBT and BST.
Thus, in this study, with the aim of elucidating the mechanism responsible for the changes in body temperature induced during swallowing, we compared the ingestion of WSE-drinks (WSD1 and WSD2) and WSE-capsule (WSC) in a randomized, double-blind, placebo-controlled, single-ingestion crossover trial. In addition, for the purpose of excluding the effects after absorption from the gastrointestinal tract into the blood and evaluating the degree of contribution of the swallowing process, our clinical trial used a lower amount of WSE than our previous study (Masuda et al., 2017). First, before the clinical trial, to maintain both the ease of swallowing the WSE-drink and blinding in the clinical trial, we evaluated the intensity of flavor (herbal and pungent) of WSD1 and WSD2 in a sensory evaluation test in Experiment (Exp.) 1. We then performed a double-blind clinical trial of WSC (in Exp. 2), WSD1 (in Exp. 3) and WSD2 (in Exp. 4).
Test samples and control samples The powdered WSE was prepared as follows. Hot water (180 g, 85 °C) was added to the dried leaves of winter savory (6 g, naturally dried leaves growing wildly in Albania), followed by stirring at 85 °C for 10 min. After cooling, the insoluble matter was removed using filter paper. The eluate was concentrated and lyophilized to obtain the powdered WSE (1.5 g, yield from winter savory leaves: 25 %).
The following test samples were used: Exp. 2, WSC, a hard capsule made of gelatin (Matsuya Corporation, Osaka, Japan) containing 40 mg powdered WSE (0.0375 mg carvacrol and 0.0075 mg thymol); Exp. 3, WSD1, a drink comprising 0.125 g liquid WSE (liquid WSE: WSE in liquid form, which was obtained from Ogawa Co., Ltd., Tokyo, Japan, 0.0375 mg carvacrol and 0.0075 mg thymol) and 50 mL of the flavored water, which was prepared by the addition of 0.1 % weight of lemon flavor (Ogawa Co., Ltd.) in water; Exp. 4, WSD2, a drink comprising 0.125 g liquid WSE (0.0375 mg carvacrol and 0.0075 mg thymol) and 25 mL of the flavored water. Because of the herbal notes and pungency of winter savory, there is the possibility of subjective effects, such as a placebo effect or a stress reaction. As previously reported (Masuda et al., 2017), lemon flavor was found to be useful for masking these characteristic flavors, therefore it was used in Exps. 3 and 4. In this study, we carried out the clinical trial using a placebo-controlled design. The following control samples were used: Exp. 2, a capsule of powdered sugar (40 mg, Ueno Sugar Co., Ltd., Osaka, Japan); Exp. 3, flavored water (50 mL); Exp. 4, flavored water (25 mL). In Exp. 2, the test and control capsules were ingested with 37 °C water (50 mL). In Exp. 3 and 4, the test and control drinks were warmed to 37 °C before ingestion.
Sensory evaluation In Exp. 1, the intensities of flavor (herbal and pungent) of WSD1, WSD2 and winter savory tea were evaluated by 13 evaluators (researchers at Ogawa Co., Ltd.; eight men and five women; age, 25-64 years). The evaluation was performed by a scoring method (Sato, 1985) in a sensory evaluation chamber under room temperature condition. A scale of 0-6 was used to measure sensory evaluation (0, not felt; 1, extremely weak; 2, weak; 3, somewhat weak; 4, somewhat strong; 5, strong; 6, extremely strong). Winter savory tea was prepared as follows: dry leaves of winter savory (1 g) were immersed in hot water (100 mL, 85 °C) for 10 min and filtered using filter paper.
Subjects for measurement of BST, CBT and blood flow Clinical trials were conducted with the approval of the Ethics Committee at the University of Shiga Prefecture in accordance with the principles of the Declaration of Helsinki. Informed consent was obtained from all subjects after a full explanation of the content of the consent form.
Subjects were selected using a questionnaire focusing on the symptoms of cold sensitivity as reported by Takumi et al. (2010). All subjects were nonsmokers and did have not chronic illness. To avoid fluctuations caused by the menstrual cycle, we performed the clinical trial during the subjects' luteal phase. Subjects were forbidden from eating and drinking anything other than water after awakening on the day of the experiment. Ingestion of alcohol or irritant foods such as spices on the day before testing was prohibited. Each subject wore the same clothing to exclude the influence of clothing on body temperature.
The test was carried out in six, seven and eight Japanese female volunteers for Exps. 2, 3 and 4, respectively. The subject characteristics (values are expressed as means ± standard deviation [SD]) were as follows: Exp. 2: age, 22 ± 1 years; height, 154.8 ± 6.8 cm; body weight, 53.8 ± 6.5 kg; and body mass index (BMI), 22.6 ± 3.3 kg/m2; Exp. 3: age, 22 ± 1 years; height, 157.6 ± 3.2 cm; body weight, 48.4 ± 4.3 kg; and BMI, 19.5 ± 1.2 kg/m2; Exp. 4: age, 22 ± 1 years; height, 160.3 ± 3.2 cm; body weight, 50.8 ± 4.5 kg; and BMI, 19.7 ± 1.5 kg/ m2.
Each measurement was taken between 09:00 and 13:00 to avoid the influence of diurnal variations in body temperature. In Exps. 3 and 4, WSD1, WSD2, or each control drink was ingested within 30 seconds. One sample was ingested on the first day and the other sample was ingested on the second day. The order of ingestion was randomized. To exclude the effects of the first sample, we arranged a washout period of >1 day. The washout period was determined with consideration of the metabolism of carvacrol and thymol (Austgulen et al., 1987; Kohlert et al., 2000 and 2002; Friedman, 2014). None of the subjects complained of discomfort after ingestion.
Test protocols for measurement of BST, CBT and blood flow This study was conducted using a randomized, double-blind, placebo-controlled, single-ingestion crossover design in a similar manner to previous reports (Masuda et al., 2011, 2013, 2016a, 2016b and 2017). Exps. 2, 3 and 4 were conducted between mid-December and early April, late September and mid-October, and mid-November and early December, respectively. To reduce the influence of seasonal changes on body temperature and to accentuate the differences in body temperature between each sample, the room temperatures were set as follows: 23 ± 0.5 °C in Exps. 2 and 4, and 24 ± 0.5 °C in Exp 3. Room humidity was maintained at approximately 50 % in all experiments.
The time-course of changes in BSTs of the wrist, middle finger, ankle, forehead and neck was measured using two thermometers (BTH-601, Bio Research Center Co., Ltd., Nagoya, Japan; AM-8000 K, Anritsu Meter Co., Ltd., Tokyo, Japan). Thermoprobes were fixed on each skin surface using surgical tape. The time-course of changes in temperature of the tympanic membrane was measured using an earplug with a thermistor thermometer (ITP010- 27, Nikkiso-Therm Co., Ltd., Tokyo, Japan). The time-course of changes in blood flow on the tip of the ring finger was measured using an ALF 21D Laser Doppler Flowmeter (Advance Co., Ltd., Tokyo, Japan). Each temperature and blood flow were measured every minute from 10 min before to 60 min after sample ingestion.
Quantification of carvacrol and thymol in WSC, WSD1 and WSD2 Quantitative analyses of carvacrol and thymol were performed using a high-performance liquid chromatography system, as described in a previous report (Masuda et al., 2011). The wavelength of 280 nm was used for quantification.
Statistical analyses The data are expressed as the means ± standard error of the mean (SEM). The data of the time-course of changes in BST, CBT and blood flow data in Exps. 2, 3 and 4 were evaluated by two-way repeated measures analysis of variance (ANOVA), using GraphPad Prism version 5 (GraphPad Software, Inc., La Jolla, CA). Comparisons between the treatment groups at each time point were evaluated using a paired t-test. The sensory evaluation data (in Exp. 1) was evaluated by one-way ANOVA with Tukey's multiple-comparison post-hoc test, using GraphPad Prism version 5. For both statistical analyses, a probability value of p < 0.05 was regarded as significant.
Sensory evaluation in Exp. 1 The intensities of flavor (herbal and pungent) of WSD1, WSD2 and herbal tea of winter savory are shown in Fig. 1. The flavor intensities of WSD1 and WSD2 were significantly less than that of winter savory tea.

Sensory evaluation of winter savory tea (Tea), WSD1 and WSD2 in Exp. 1.
Values are expressed as means ± SD. n = 13. Test scales: 0, not felt; 1, extremely weak; 2, weak; 3, somewhat weak; 4, somewhat strong; 5, strong; 6, extremely strong. Values with different superscript letters are significant difference at p < 0.05 (one-way ANOVA with Tukey's multiple-comparison post-hoc test).
Time-course of changes in BST, CBT and blood flow after ingestion of WSC (in Exp. 2) The vertical axis shows the changes in body temperature, with the temperature at 0 min as the base point. There were no significant differences in the time-course of changes in BSTs at the wrist, finger, ankle, toe, forehead and neck, and CBT, as measured at the tympanic membrane (Childs et al., 1999), between the WSC- and the control-ingestion groups (Fig. 2A–G).
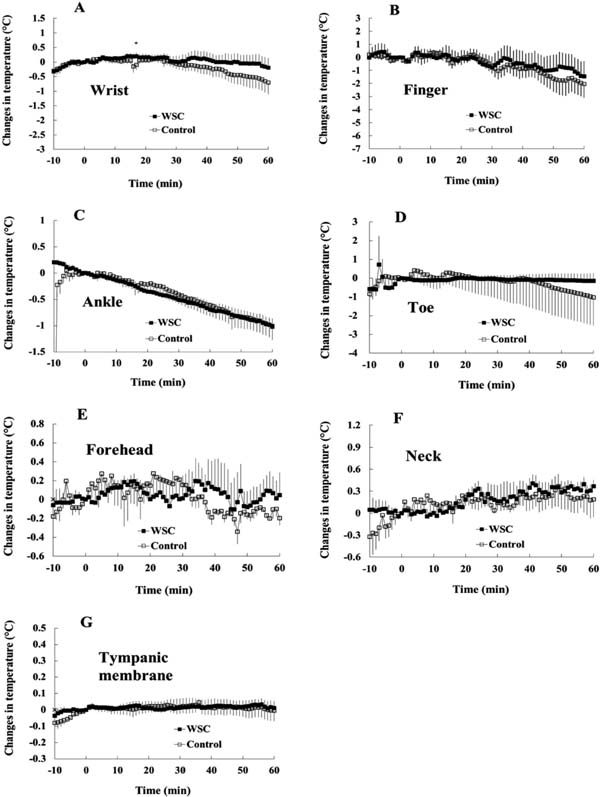
Time-course of changes in body temperature at the wrist (A), finger (B), ankle (C), toe (D), forehead (E), neck (F) and tympanic membrane (G) before and after the ingestion of WSC (a capsule composed of 40 mg powdered WSE) or control in Exp. 2.
Values are expressed as means ± SEM. n = 6. * indicates p < 0.05 (paired t-test).
There was no significant difference in the changes in blood flow of the finger between the WSC- and the control-ingestion groups (Fig. 3).

Time-course of changes in blood flow of the finger before and after the ingestion of WSC (a capsule composed of 40 mg powdered WSE) or control in Exp. 2.
Values are expressed as means ± SEM. n = 6. * indicates p < 0.05 (paired t-test).
Time-course of changes in BST, CBT and blood flow after ingestion of WSD1 (in Exp. 3) The BSTs of the wrist and finger after the ingestion of WSD1 were significantly higher than those after the ingestion of the control in Exp. 3 (Fig. 4A and B). However, for other body parts, such as the ankle, toe, forehead, neck and tympanic membrane, no significant differences between the WSD1- and the control-ingestion groups were observed (Fig. 4C–G). Significant differences were observed in the changes in blood flow of the finger between the WSD1- and the control-ingestion groups (Fig. 5).

Time-course of changes in body temperature at the wrist (A), finger (B), ankle (C), toe (D), forehead (E), neck (F) and tympanic membrane (G) before and after the ingestion of WSD1 (a drink comprising 0.25 % liquid WSE) or control in Exp. 3
Values are expressed as means ± SEM. n = 7, § indicates p < 0.05 at the wrist and finger for −10 to 60 min (two-way repeated measures ANOVA). * indicates p < 0.05 (paired t-test).

Time-course of changes in blood flow of the finger before and after the ingestion of WSD1 (a drink comprising 0.25 % liquid WSE) or control in Exp. 3.
Values are expressed as means ± SEM. n = 7, § indicates p < 0.05 for −10 to 60 min (two-way repeated measures ANOVA). * indicates p < 0.05 (paired t-test).
Time-course of changes in BST, CBT and blood flow after ingestion of WSD2 (in Exp. 4) There were significant differences between the WSD2- and the control-ingestion groups in changes in BSTs at the wrist, finger, toe, forehead and neck (Fig. 6A, B and D–F), and CBT at the tympanic membrane (Fig. 6G).

Time-course of changes in body temperature at the wrist (A), finger (B), ankle (C), toe (D), forehead (E), neck (F) and tympanic membrane (G) before and after the ingestion of WSD2 (a drink comprising 0.5 % liquid WSE) or control in Exp. 4.
Values are expressed as means ± SEM. n = 8, § indicates p < 0.05 at the wrist, finger, toe, forehead, neck and tympanic membrane for −10 to 60 min (two-way repeated measures ANOVA). * indicates p < 0.05 (paired t-test).
The changes in the blood flow of the finger after the ingestion of WSD2 was significantly higher than that after the ingestion of the control (Fig. 7).

Time-course of changes in blood flow of the finger before and after the ingestion of WSD2 (a drink comprising 0.5 % liquid WSE) or control in Exp. 4.
Values are expressed as means ± SEM. n = 8, § indicates p < 0.05 for −10 to 60 min (two-way repeated measures ANOVA). * indicates p < 0.05 (paired t-test).
The mean of changes in BST and CBT between WSC, WSD1 or WSD2, and each control sample (in Exps. 2, 3 and 4) The mean of the changes in BST and CBT for 60 min between the test samples (WSC, WSD1 or WSD2) and each control sample are shown in Fig. 8. The vertical axis shows the mean of the changes in body temperature, with 0 min as a base point. The extent of the change in each sample (WSC, WSD1 or WSD2) was larger in the finger than in other body parts.

Mean of changes in body temperature between the test samples (WSC (a capsule composed of 40 mg of powdered WSE), WSD1 (a drink comprising 0.25 % liquid WSE) or WSD2 (a drink comprising 0.5 % liquid WSE)), and each control sample in Exps. 2, 3 and 4.
Values are expressed as means ± SEM (n = 6, 7 and 8 in Exps. 2, 3 and 4, respectively). § indicates significant differences (p < 0.05 by two-way repeated measures ANOVA) in the time-course of changes in body temperature between the test samples (WSD1 or WSD2) and each control sample shown in Fig. 4 and 6.
It is possible that the difficulty of ingestion owing to an irritant flavor might induce a stress reaction (Sugimoto et al., 2013); therefore, prior to Exps. 3 and 4, we performed a sensory evaluation (Fig. 1). The flavor intensities of WSD1 and WSD2 were confirmed to be lower than those of winter savoy tea. As a result, all subjects in Exps. 3 and 4 did not recognize the differences between the test sample (WSD1 or WSD2) and each control sample (data not shown). Thus, the blindness in the double-blind trial using WSD1 (Exp. 3) and WSD2 (Exp. 4) was confirmed. Moreover, given the removal of bias, all staff and subjects were not notified about the contents of the sample.
It has been reported that active principles in WSE (carvacrol and thymol) might induce the activation of gastrointestinal TRP channels, followed by the activation of the gastrointestinal vagus nerve and changes in body temperature, without absorption from the gastrointestinal tract into the blood (Masuda et al., 2016b). In this study, as shown in Fig. 2, after the ingestion of WSC (which dissolved in the stomach), neither CBT (tympanic membrane) nor BSTs (wrist, finger, ankle, toe, forehead and neck) significantly increased compared with those after the ingestion of the control. Therefore, it is suggested that the stomach and the intestines were not involved in changes in temperature at this amount of WSE. On the other hand, as shown in Fig. 4, the ingestion of WSD1 (which is composed of WSE-drink containing an equal amount of active principles) induced a significant increase in peripheral BSTs (wrist and finger) compared to that of the control, but not in CBT (tympanic membrane) and BSTs (forehead and neck; which have been reported to show similar movements to the tympanic membrane (Ikeda et al., 1997)). As for the site of action, given that there is a difference (oral cavity, throat and esophagus) between the ingestion of WSE-drink (oral cavity, throat, esophagus, stomach and intestines) and that of WSE-capsule (stomach and intestines), it is suggested that the swallowing process (oral cavity, throat and esophagus) after the ingestion of WSD1 might greatly contribute to heat transfer to peripheral sites (wrist and finger) caused by peripheral vasodilation regardless of heat production in the core body.
TRP vanilloid 1 (TRPV1) or TRP ankyrin 1 (TRPA1) is thought to induce not only heat production via the central nervous system (which is greatly related to thermoregulation) (Watanabe et al., 1988; Caterina, 2007; Iwasaki et al., 2008), but also the vasodilation of peripheral arteries via the release of calcitonine gene-related peptide (CGRP), a potent vasodilator neuropeptide (Caterina, 2007; Earley, 2012; Zygmunt and Högestâtt, 2014). As for CGRP, it has been reported that Raynaud's phenomenon, a peripheral circulation disorder, involves a deficiency in CGRP-containing perivascular nerves in finger skin (Bunker et al., 1996). According to a report by Kawasaki and Takahashi (1993), CGRP might be released via the TRP channel activation and involvement of the central nervous system. Thus, given that WSE can activate TRPV1 and TRPA1 channels using human TRPV1- and human TRPA1-expressing cells, respectively (data not shown), there is a possibility that the activation of TRP channels (which are expressed at free nerve endings of the afferent nerve in the oral cavity, throat and esophagus (Miwa et al., 2010; Peyrot des Gachons et al., 2011; Alvarez-Berdugo et al., 2016)) during swallowing might induce a signal transduction to the central nervous system, followed by the vasodilation of peripheral arteries, heat transfer and the inhibition of the decrease in BSTs (wrist and finger). However, studies elucidating signal transduction to the central nervous system and the release of CGRP (which occur by the activation of TRP channels) are needed.
As shown in Fig. 4 and 6, higher the concentration of WSE-drink was (WSD1 < WSD2), greater the increase in CBT (tympanic membrane) became. Given that the stronger the stimulation, the greater the nerve response (Schmidt, 1989), it is suggested that the increase in nerve response (which was caused by the increase in WSE concentration) might contribute to heat production. Next, we summarized the results from this and previous studies (Masuda et al., 2017) in Table 1. As shown in Table 1 (No. 2 and 3), in the comparison of WSD2 with DK1 (DK1 belongs to WSE-drink; WSE amount in DK1 was twice that in WSD2, but WSE concentration in DK1 was only half that in WSD2), WSD2 induced a significant increase in CBT (tympanic membrane), but DK1 did not. As also shown in Table 1 (No. 1 and 2), a significant increase in CBT (tympanic membrane) was observed in response to the increase in WSE concentration. Therefore, to increase CBT (tympanic membrane) with the ingestion of WSE-drink, an increase in WSE concentration might be more effective than an increase in WSE amount.
| No. | WSE-drink | Amount of liquid WSE in WSE-drink (mg) | Concentration of liquid WSE in WSE-drink (%) | Significant differences (§ indicates p < 0.05 by two-way repeated measures ANOVA) in the time-course of changes in body temperature between WSE-drink (WSD1, WSD2 or DK1) and each control sample | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Wrist | Finger | Ankle | Toe | Forehead | Neck | Tympanic membrane | ||||
| 1 | WSD1 | 125 2) | 0.25 | § | § | |||||
| 2 | WSD2 | 125 2) | 0.5 | § | § | § | § | § | § | |
| 3 | DK1 1) | 250 3) | 0.25 | § | § | § | § | § | ||
As for heat production, carvacrol was reported to be a representatively active principle in WSE (Masuda et al., 2013). It is supposed that the TRPA1 channel was activated by carvacrol, followed by signal transduction to the central nervous system and heat production in brown adipose tissue (Iwasaki et al., 2008; Masamoto et al., 2009). In this study, the activation of TRPA1 in the swallowing process (oral cavity, throat and esophagus) by carvacrol in WSE-drink might have induced signal transduction to the central nervous system, followed by heat production and the increase in CBT (tympanic membrane). However, further study using the TRPA1 antagonist is needed to elucidate this point more clearly.
The extent of the changes in body temperature after the ingestion of WSD1 or WSD2 was greater in the finger than in other body parts (Fig. 8). Moreover, the ingestion of WSD2 produced remarkable changes in the finger, toe and wrist. Given that TRP activation might greatly contribute to heat production (leading to the increase in CBT) and heat transfer (leading to the inhibition of the decrease in BST), it is suggested that WSE-drink might be effective not only in humans who experience cold sensitivity (Terasawa, 1987; Imai et al., 2007), but also healthy persons.
In conclusion, it is suggested that neurostimulation from the swallowing process (oral cavity, throat and esophagus) after drinking WSE might greatly contribute to heat production (leading to the increase in CBT) and heat transfer (caused by peripheral vasodilation leading to the inhibition of the decrease in BST at peripheral site) independently of each other. In addition, it is suggested that the increase in WSE concentration in drink might contribute to heat production compared to the increase in WSE amount in drink.
Acknowledgments We are grateful to Ms. Satsuki Inagaki and Dr. Shu Kaneko of Ogawa & Company, Ltd. for their technical assistance, the sensory evaluators of Ogawa & Company, Ltd. and the subjects of the clinical trials of the University of Shiga Prefecture for their cooperation.