2019 年 25 巻 3 号 p. 479-484
2019 年 25 巻 3 号 p. 479-484
Escherichia coli cells suspended in phosphate-buffered saline (PBS) were treated by high hydrostatic pressure (HHP; 400 – 600 MPa) at 25 °C for 10 min and then stored at 5 – 25 °C. When treated at 600 MPa, the cells were inactivated lethally. Treatment at 400 and 500 MPa reduced viable cell counts by approximately 7 and 8 log, respectively. Viable counts of cells treated at 400 or 500 MPa increased obviously during storage at 15 and 25 °C, whereas they did not increase at 5 and 10 °C. Meanwhile, healthy E. coli cells inoculated to a heat-killed dead cell suspension in PBS showed drastic growth during storage at 15 and 25 °C, but no growth at 5 and 10 °C. Therefore, the obvious increase in viable counts of HHP-treated cells might be attributed to their cannibalism of the dead cells.
To ensure food safety during food processing, intervention technologies such as heating, ultraviolet irradiation, and sanitizer treatment are applied to induce lethal and sublethal damage to various physiological functions of bacteria. Survivors of intervention are termed sublethally inactivated bacteria or injured bacteria (Noriega et al., 2013; Perni et al., 2013; Zhao et al., 2013; Gayán et al., 2014; Zheng et al., 2015). They are vulnerable to heat, high hydrostatic pressure (HHP), oxidation, and other stresses as compared with healthy bacteria (Dodd et al., 2007; Koseki et al., 2008; McKenzie et al., 2014). In addition, their ability to form colonies on agar plates is conditional and is dependent on environmental factors such as temperature, due to damaged physiological functions (Blackburn et al., 2000). Therefore, some conventional methods for microbial detection such as direct plating culture method may not suitably evaluate viable counts of injured bacteria. Culture methods under specific conditions may allow detection of injured bacteria via promoted recovery to healthy bacteria capable of forming colonies on agar plates (Chawla et al., 1996; Smelt et al., 2002; Miller et al., 2006; Jasson et al., 2009; Smigic et al., 2009; Bi et al., 2015). However, conditions for the recovery of injured bacteria and the responsible mechanisms are not fully understood.
HHP has been applied to food processing for bacterial inactivation, and HHP-processed foods have been distributed and stored under refrigerated conditions (Smelt et al., 2002; Yamamoto, 2017). However, it has been pointed out that HHP-treated bacteria may not be detected immediately after HHP treatment, while HHP-injured bacteria have been studied intensively to reveal their recovery behaviors during storage of HHP-processed food and liquid food models (Bozoglu et al., 2004; Bull et al., 2005; Chilton et al., 2001, Koseki and Yamamoto, 2006; Koseki et al., 2008; Inaoka et al., 2017; Kimura et al., 2017).
Experimental models of liquid and solid foods have been indispensable in facilitating basic understanding of HHP-injured bacteria. Suspension of Escherichia coli in phosphate-buffered saline (PBS) has been used conventionally as a typical nutrient-free experimental model as well as a liquid food model, and it has been considered useful to clarify the behavior of HHP-injured E. coli in nutrient-free beverages such as mineral water.
In the detection of HHP-treated bacteria by direct plating method, incubation temperature plays a critical role (Koseki and Yamamoto, 2006). In our previous studies, the incubation temperature was typically set at 37 °C, which is the optimal growth temperature for E. coli (Salter et al., 1998). However, it was reported that HHP-injured populations were partly or totally killed with incubation at 37 °C (Koseki and Yamamoto, 2006; Koseki et al., 2008; Kimura et al., 2017).
When HHP-treated cells of E. coli ATCC25922 in PBS (400 – 600 MPa, 25 °C, 10 min) were stored at 25 or 37 °C and then surface-plated for incubation at 37 °C, cells were either detectable or nondetectable depending on the storage temperature and the HHP level (Koseki and Yamamoto, 2006). When treated at 500 MPa or higher, the cells were nondetectable throughout the storage at 37 °C, while nondetectable cells became detectable during storage at 25 °C. This suggests that the HHP-injured cells were nondetectable at the early stage of storage at 25 °C and then became tolerant of 37 °C incubation. This supports the incubation-induced death of HHP-injured cells at 37 °C. On the other hand, 400 MPa-treated cells were detectable throughout storage at 25 and 37 °C, suggesting that healthy survivors were able to grow at both temperatures.
In addition, E. coli ATCC25922 cells in PBS treated at 400 or 500 MPa (25 °C, 10 min) were more highly detected at 25 °C than at 37 °C on LB plates (Kimura et al., 2017). This report also supports the incubation-induced death of HHP-injured cells during 37 °C incubation. Furthermore, storage-induced death of HHP-treated bacteria was supported by a report on HHP-treated (550 MPa, 25 °C, 5 min) Listeria monocytogenes cells in milk subsequently stored at 37 °C. Cells were detectable during storage at 37 °C for 2 h and then became nondetectable at 4 h (Koseki et al., 2008).
It was reported that HHP treatment (500–600 MPa, 25 °C, 10 min) inactivated E. coli ATCC25922 (of slightly higher than 8 log CFU/mL; hereafter, log) in PBS to nondetectable levels (Koseki and Yamamoto, 2006). On the other hand, subsequent increase in cell counts was also observed, reaching slightly lower than 8 log during storage at 25 °C (Koseki and Yamamoto, 2006). From the results, two explanations can be suggested. The increase in viable cell counts might be ascribed to a full recovery to ca. 8 log without cell proliferation. Otherwise, a partial recovery (e.g., by 1–3 digits) was followed by cell proliferation to ca. 8 log. In the latter case, a small population of either healthy or injured survivors of E. coli could utilize HHP-inactivated dead cells as a nutrient source; this is known as cannibalism, which is defined as “the eating of the flesh of an animal by another animal of the same kind” in animals. In general, dead cells can be utilized as a nutrient source for bacterial growth (Guo et al., 2013). However, the cannibalistic growth of E. coli cells has not been sufficiently clarified, especially in terms of their recovery from HHP-induced injury.
In this study, the recovery behavior of HHP-injured E. coli in a nutrient-free liquid food model (PBS) was studied, assuming food storage at selected temperatures between 5 °C (refrigeration) and 25 °C (ambient temperature). Viable cell counts were evaluated by direct plating method at an incubation temperature of 37 °C, where detection of HHP-injured cells of E. coli is assumed to be minimized. Cell recovery was discussed from the viewpoint of cannibalistic growth.
Bacterial strain and growth conditions Escherichia coli (ATCC25922) was purchased from the American Tissue Culture Collection (Manassas, VA, USA). The bacterial strain was stored at −80 °C in a stock mixture (1 part 80% glycerol to 3 parts 0.9% NaCl aqueous solution). The frozen stock was thawed and inoculated in 100 mL of trypticase soy broth (TSB; Nissui Pharmaceutical Co., Ltd., Tokyo, Japan). The culture was incubated at 37 °C with 130 rpm of agitation for 18 h. Cells at the stationary phase were collected by centrifugation (2,300 g, 5 °C, 10 min). The precipitated pellet was washed, suspended in PBS, centrifuged as described above, and decanted. The washing procedure was repeated twice. Then, the resulting precipitate was resuspended in 200 mL of PBS (pH 7.4), and 60 mL of the bacterial suspension was heat-sealed into a sterile polypropylene bag. The bacterial suspension was also used to prepare a heat-killed dead cell suspension, and a healthy cell suspension for inoculation to the dead cell suspension.
HHP treatment Bacterial suspensions were subjected to HHP treatment using a HHP processor (Dr. Chef, Kobe Steel Ltd., Kobe, Japan). The pressure chamber (60 mmΦ × 200 mm) was filled with water as a pressure medium, whose temperature was set at 25 °C using a temperature controller, with circulated refrigerant around the chamber. A sample bag was immersed in the chamber, pressurized up to 400, 500, or 600 MPa at a compression rate of 200 MPa/min, held for 10 min, and decompressed to ambient pressure at 200 MPa/min. The HHP-treated bacterial suspension was dispensed into 4 sterile capped polypropylene tubes, and the tubes were incubated at 5, 10, 15, and 25 °C for at least 240 h.
Bacterial growth by utilizing heat-killed dead cells The suspension of heat-killed dead cells, which has been confirmed as a nutrient source for cannibalistic growth (Nioh and Furusaka, 1968), was employed to study the cannibalistic growth of E. coli cells. The bacterial suspension in the bag (9.1 ± 0.1 log) was immersed in a hot water bath at 90 °C for 30 min and then cooled to ambient temperature using running water. No survivors in the suspension were detected immediately after the heat treatment as well as during storage by direct plating (< 0.6 log CFU/mL or 4 CFU/mL). After cooling, stationary-phase healthy cells were inoculated to the PBS suspension of the heat-killed cells at a final level of 2 log, and the mixture was dispensed into sterile capped polypropylene tubes for storage at 5, 10, 15, and 25 °C for at least 240 h.
Enumeration of bacterial cells Viable bacterial cells in the sample suspension were enumerated by direct plating method on standard method agar (Nissui Pharmaceutical Co., Ltd.). A portion (250 µL) of the suspension was spread on the plate in quadruplicate without dilution; the remainder was serially diluted using sterile physiological saline (0.9% NaCl aqueous solution), and a portion (100 µL) of the serially diluted solution was spread on the plate in triplicate. The agar plates were incubated for 24 h at 37 °C, where HHP-injured populations were assumed to be either killed or growth-suppressed during incubation (Koseki and Yamamoto, 2006; Koseki et al., 2008; Kimura et al., 2017). All experiments were conducted twice independently. Plate count data were expressed as mean ± standard deviation.
Bacterial behaviour immediately after HHP treatment Immediately after HHP treatment, viable cell counts of E. coli varied depending on the level of HHP (Fig. 1). Immediately after HHP treatment at 400 MPa (Fig. 1), the viable cell count was 2.2 ± 0.9 log, which was ca. 1 log higher than that after 500 MPa treatment (1.3 ± 0.7 log). As for the viable cell count immediately after 600 MPa treatment, no survivors were detected (< 0.6 log CFU/mL or 4 CFU/mL). Thus, fewer survivors were observed at higher levels of HHP. Furthermore, the survivors may have included healthy and/or injured cells.

Effect of pressure level on the survivors immediately after HHP treatment of E. coli cells in PBS. HHP treatment was carried out at 25 °C for 10 min. Data were obtained by plating in triplicate and expressed as mean of two independent trials ± standard deviation. N.D., not detected.
HHP treatment at 400 MPa reduced the viable cell count of E. coli from 9.1 ± 0.0 log to 2.2 ± 0.9 log, as shown in Fig. 1. Thereafter, the viable cell count changed with different trends depending on the storage temperature (Fig. 2). During storage at 5 and 10 °C, viable cell counts increased temporarily at the early stage, with maximal counts of 2.9 ± 0.5 log at 120 h and 3.8 ± 0.4 log at 72 h, respectively. Thereafter, the viable cell counts decreased gradually, possibly indicating the death phase.

Recovery of 400 MPa-treated E. coli cells in PBS during storage at 5 °C, 10 °C, 15 °C, and 25 °C. Data were obtained by plating in triplicate and expressed as mean of two independent trials ± standard deviation.
On the other hand, for storage at 15 and 25 °C, the viable cell counts drastically increased to ca. 8 log. During storage at 15 °C, a biphasic growth curve was observed, with a broad peak of temporary increment until 144 h and a subsequent exponential increase. A maximum of 4.2 ± 0.4 log was observed at 36 h. During storage at 25 °C, the viable cell counts increased almost immediately after treatment and showed a trace shoulder, indicating that the temporary increase represents a short lag phase.
After 500 MPa treatment, the growth curve at 5 or 10 °C (Fig. 3) was comparable with that at 10 °C after 400 MPa treatment (Fig. 2). Maxima of 1.9 ± 0.2 log at 96 h and 2.3 ± 0.3 log at 120 h were observed during storage at 5 and 10 °C, respectively. Thereafter, the cells at these storage temperatures seemed to enter the death phase, leading to almost nondetectable viable cell counts (< 0.6 log).
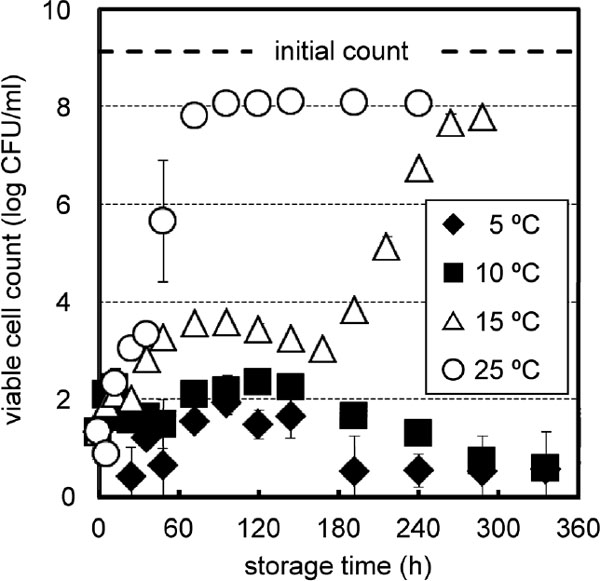
Recovery of 500 MPa-treated E. coli cells in PBS during storage at 5, 10, 15 and 25 °C. Data were obtained by plating in triplicate and expressed as mean of two independent trials ± standard deviation.
The viable cell counts after 500 MPa treatment during storage at 15 and 25 °C (Fig. 3) followed similar trends to those after 400 MPa treatment (Fig. 2), respectively. After short and very short lag phases, the viable cell counts at 15 and 25 °C approached ca. 8 log (7.8 ± 0.0 and 8.1 ± 0.0 log), respectively. During storage at 15 °C, a biphasic growth curve was observed as in the case of 400 MPa treatment, showing a broad peak of temporary increment until 168 h and a subsequent exponential increase (Fig. 3). A maximum of 3.6 ± 0.1 log was observed at 96 h. As in the case of 400 MPa treatment, there was a similar tendency where peak values appeared earlier with increasing storage temperature of 5, 10, and 15 °C. Furthermore, a longer storage period was needed after 500 MPa treatment to reach the maximum at each storage temperature than after 400 MPa treatment.
Treatment at 600 MPa reduced the E. coli (9.1 ± 0.0 log) count to a nondetectable level (< 0.6 log CFU/mL or 4 CFU/mL), which was confirmed during storage at 5, 10, 15, and 25 °C up to 10 d (data not shown). HHP treatment at 600 MPa may have either inactivated E. coli cells lethally or suppressed their recovery completely.
Cannibalistic growth of E. coli in dead cell suspension in PBS A dead cell suspension was prepared by heat treatment, which decreased the viable count of E. coli (9.1 ± 0.1 log) to a nondetectable level (< 0.6 log CFU/mL or 4 CFU/mL). Viable healthy E. coli cells at the stationary phase were then inoculated to the suspension at a level of 2.1 ± 0.0 log, and the inoculated suspensions were subsequently stored at 5, 10, 15, and 25 °C.
Viable counts of E. coli did not increase at 5 and 10 °C, while increased drastically at 15 and 25 °C (Fig. 4).

Effect of storage temperature and time on the viable count of healthy E. coli cells in PBS containing their heat-killed dead cells. No HHP treatment was applied to the cells. Data were obtained by plating in triplicate and were expressed as mean of two independent trials ± standard deviation.
Viable cell counts were almost constant at 5 °C, but they showed a gradual decrease at 10 °C. It is possible that the cells entered the death phase at 10 °C due to starvation (Lappin-Scott and Costerton, 1990); however, the mechanism of cell death is not clear at this stage. When E. coli cells were suspended in nutrient-rich TSB, the cells did not grow at 5 and 10 °C for 10 d (data not shown). This indicated that the healthy E. coli cells did not utilize the dead cells or nutrients in TSB for growth at 5 and 10 °C. It can be speculated that the E. coli cells that did not grow at 5 and 10 °C in the presence of dead cells or nutrients in TSB might enter the death phase due to starvation, which might explain the decreases in viable counts of cells treated with 400 and 500 MPa and stored at 5 and 10 °C.
On the other hand, during storage at 15 and 25 °C, viable counts of E. coli increased up to ca. 7 log, which was approximately 1% of the number of heat-killed dead cells in PBS. A similar growth curve to Fig. 4 was reported for E. coli grown at 30 °C in water containing their heat-killed cells, indicating the role of dead cells as a nutrient source (Nioh and Furusaka, 1968). It is considered to be cannibalism when healthy E. coli cells consume dead cells or their components as nutrients for growth. Therefore, it was suggested in this study that the drastic increase in viable cell counts up to ca. 7 log during storage at 15 and 25 °C was also due to cannibalism.
Recovery and cannibalistic growth of HHP-injured E. coli cells No cannibalistic growth was observed at 5 and 10 °C for healthy cells in the heat-killed dead cell suspension (Fig. 4). Meanwhile, viable cell counts increased temporarily at the early stage of storage at 5 and 10 °C in the HHP-treated cell systems (Figs. 2 and 3). It is known that elevated temperatures affect almost every cellular structure, through membrane damage, loss of nutrients and ions, ribosome aggregation, DNA strand breakage, inactivation of essential enzymes, and protein coagulation; whereas, HHP has limited effects on cells via damage to DNA, the envelope, ribosomes, and other proteins (Mañas and Pagán, 2005). In addition, it was reported that pressure-treated stationary-phase E. coli cells may maintain a physically intact cytoplasmic membrane upon decompression, even when dead (Pagán and Mackey, 2000; Mañas and Mackey, 2004). It is likely that the availability of HHP-killed E. coli cells as a nutrient source for healthy cells would be lower than that of heat-killed cells.
The cannibalistic growth of healthy cells in HHP-killed cells was not assessed in this study. However, since healthy cells did not proliferate in the heat-killed dead cell suspension during storage at 5 and 10 °C (Fig. 4), it is likely that the temporary increments were not due to cannibalistic growth on HHP-killed cells, which are probably less available than heat-killed cells as discussed above. Therefore, the temporary increments may be ascribed to the recovery of colony forming ability in HHP-injured cells, which were not detected before the temporary increments because of the inability to form colonies. Regardless of whether the temporary increments were due to recovery of colony forming ability or not, the live cells during the temporary increments should consume nutrients, either as cellular components derived from the HHP-killed dead cells or intracellular components of HHP-injured cells. However, since the healthy cells did not grow on the heat-killed dead cells at 5 and 10 °C (Fig. 4), it can be speculated that HHP-injured cells would consume their own intracellular components for the temporary recovery at 5 and 10 °C rather than HHP-killed cells. Further investigation is necessary to clarify the mechanism of the temporary increments regarding the consumption of intracellular components.Temporary increments as lag phases of ca. 144 h and 168 h were respectively observed for cells treated with 400 and 500 MPa during storage at 15 °C (Figs. 2 and 3). The lag phases appeared as temporary increments before exponential increases in viable counts, while healthy cells in the heat-killed dead cell suspension showed a spontaneous increase at 15 °C without a notable lag phase (Fig. 4). This observation is in agreement with previous reports, in which the lag phase in bacterial growth can be longer for injured bacteria than for healthy ones (Smelt et al., 2002; Jasson et al., 2009; Smigic et al., 2009).
As discussed regarding HHP-injured cells during storage at 5 and 10 °C, cells treated with 400 and 500 MPa and stored at 15 °C would also consume their own intracellular components rather than those of HHP-killed cells for the temporary increments as the lag phases. Further study is necessary to prove this point.
Cannibalism was observed at 15 and 25 °C with healthy E. coli cells in the suspension of heat-killed dead cells (Fig. 4). Accordingly, cannibalistic growth may explain the drastic increases in the viable counts of HHP-treated cells during storage at 15 and 25 °C (Figs. 2 and 3).
During storage at 15 and 25 °C after 400 and 500 MPa treatments, the initial viable cell counts were 2.2 ± 0.9 and 1.3 ± 0.7 log, respectively, and both counts plateaued at ca. 8 log, which was 1 digit lower than the count before the HHP treatments (ca. 9 log). Accordingly, it can be calculated that injured survivors of ca. 1 to 2 log after 400 and 500 MPa treatments would have consumed HHP-killed cells of ca. 9 log to grow up to ca. 8 log cells. This trend for HHP-treated cells was comparable with the case of cannibalistic growth of healthy cells in the dead cell suspension (Fig. 4). The availability of HHP-treated dead cells for cannibalistic growth at 15 and 25 °C might be comparable with that of heat-killed cells.
HHP-treated E. coli cells in PBS were either dead or injured depending on the HHP level. The recovery of HHP-injured E. coli cells in PBS was evaluated from the view point of cannibalistic growth. The cannibalistic growth of healthy cells in the dead cell PBS suspension was observed at 15 and 25 °C, but not at 5 and 10 °C. Thus, the storage temperature after HHP treatment is a critical factor in the likelihood of cannibalistic growth after recovery. Injury induced by other intervention technologies such as heating, chemical treatment, and irradiation may differ from that by HHP treatment. Therefore, attention should be paid when applying our results to understanding non-HHP injuries.
The results of this study were obtained using PBS as the liquid food model. PBS is a nutrient-free medium containing only inorganic compounds. However, it is possible that certain food components may have a protective role in HHP-induced bacterial inactivation (Patterson et al., 1995; García-Graells et al., 2000; Buzrul et al., 2008) or be available as nutrients to promote recovery (Koseki and Yamamoto, 2006; Pagán et al., 2001). Therefore, it is necessary to further study the effect of storage temperature on the recovery of HHP-injured bacteria in nutrient-rich food systems to ensure their control during the distribution and storage of HHP-processed food.
Acknowledgments This work was supported by JSPS KAKENHI Grant Number JP15K18762.