2019 Volume 25 Issue 4 Pages 555-562
2019 Volume 25 Issue 4 Pages 555-562
The thermal and acid stability of Oryza sativa rice albumin (RA), which suppresses postprandial hyperglycaemia, was evaluated for its use in foods. RA showed high thermal stability, with a peak temperature (Tp) of 100.8 °C by differential scanning calorimetry measurement. However, its Tp shifted to 52.0 °C with the addition of dithiothreitol, suggesting that the heat resistance of RA is attributed to its rigid structure supported by intermolecular disulphide bonds. The α-amylase inhibitory activity of RA was retained even after heating to 100 °C, although it gradually decreased with longer heating times or increasing concentrations of RA. RA showed high nitrogen solubility and surface properties, such as emulsifying and foaming properties, even under acidic conditions, as opposed to other common food proteins (i.e., casein, soy protein isolate, and dried egg white). These findings suggest that RA can be used as an additive in various foods.
Rice (Oryza sativa) is a staple food in many countries, and is one of the most widely consumed plant foods serving as a principal source of carbohydrates and protein in Asia (Kennedy and Burlingame, 2003). Rice grains contain proteins that account for 8% (w/w) of the dried starchy endosperm, most of which accumulates in protein bodies (Ogawa et al., 1987). Among cereal proteins, rice protein ranks high in nutritive quality, being rich in the essential amino acid lysine (Bean and Nishita, 1985). The protein composition of rice grains is 80% glutelins (acid-alkaline-soluble), 12% globulins (salt-soluble), 5% albumins (water-soluble), and 3% prolamins (alcohol-soluble) (Juliano, 1994). Some of these proteins have beneficial physiochemical functions, such as the hypocholesterolaemic effects of prolamins (Yang et al., 2007) and globulin (Tong et al., 2012) and glutelin's ameliorating effect on diabetic nephropathy progression (Kubota et al., 2013). Although rice albumin (RA) has been less studied compared with glutelin and globulin, our previous study demonstrated that RA possesses potent hypoglycaemic activity (Ina et al., 2016). As RA is fairly resistant to protease digestion, it may suppress glucose absorption from the small intestine via a similar action as dietary fibre. Thus, RA could be used as a food material to treat postprandial hyperglycaemia, even when starch- or glucose-based foods are ingested.
According to an analysis of RA by SWISS-MODEL using the structure of wheat albumin as a template, the RA molecule is assumed to have five disulphide bonds (Ina et al., 2016), making its structure rather tight and thus reducing its digestibility. Proteins with a tight structure are also expected to be resistant to denaturation by heating and acid treatment. Therefore, in the present study, we evaluated the contribution of disulphide bonds to the thermal stability of RA.
In considering the use of protein as a food material, other physicochemical characteristics, such as solubility and functional (e.g., foaming and emulsifying) properties, are also highly important to thermal stability (Wang et al., 1999). As RA is water-soluble, tasteless, and odourless, it can be added to almost any type of food, including beverages. Moreover, RA can be easily extracted into water during the washing of rice before cooking, as it mainly exists on the surface of the rice grain. Therefore, if RA retains high solubility in water and possesses high foaming and emulsifying properties over a wide pH range, it could be applied to foods of various forms.
The importance of protein physicochemical properties is well recognized and has been investigated for some food proteins (Ahmedna et al., 1999; Aluko and Yada, 1995; Tomotake et al., 2002; Kinsella, 1979). However, physicochemical characteristics may change after processing and sterilization, as proteins can lose functionality due to their denaturation during heating and acid treatment. Therefore, another aim of the present study was to evaluate the water solubility and functional properties of RA in the pH range of 3 to 8, which are typical pH values of foods, for its application as a healthful functional food material. The functional properties of RA were compared to those of casein, soy protein isolate (SPI), and dried egg white (DEW), which are commonly used in the food industry (Kinsella, 1984; Yasumatsu et al., 1972; Campbell et al., 2003).
Experimental materials RA (85–95% protein) was prepared from rice grains (Oryza sativa japonica cv. Nipponbare) according to previously described methods (Ina et al., 2016). SPI (90% protein), DEW (94% protein), and casein (83% protein) were purchased from Fuji Oil Co., Ltd. (Osaka, Japan), Kewpie Corp. (Tokyo, Japan), and Wako Pure Chemical Industries, Ltd. (Osaka, Japan), respectively.
Thermal stability The contribution of disulphide bonds to thermal stability was evaluated by measuring the denaturation temperature of RA with or without the addition of a reducing agent using a differential scanning calorimeter (DSC-100; Seiko Instruments Inc., Chiba, Japan). RA was dissolved in water or 200 mM dithiothreitol (DTT) solution to 20% (w/w) and then stirred for 16 hr at room temperature. Then, 30 mg of each RA solution (with or without DTT) was weighed into silver pans. The pans were hermetically sealed and heated from 25 to 130 °C at a rate of 1 °C/min. A sealed pan containing the same volume of distilled water or 200 mM DTT solution was used as a reference. Peak temperature (Tp) and enthalpy of denaturation (ΔH) were computed from the thermograms using Standard Analysis (Seiko Instruments Inc.).
The thermal stability of RA was evaluated by measuring its inhibitory activity against α-amylase from mealworms after heating. RA samples were dissolved in 1 mL distilled water to 0.1% (w/w), stirred for 60 min at room temperature, and boiled at 100 °C for 10–120 min. After cooling to room temperature, α-amylase inhibitory activity was measured as previously described (Ina et al., 2016). Thermal stability was measured as the ratio of remaining α-amylase inhibitory activity of RA after heat treatment and calculated as follows: thermal stability (%) = (α-amylase inhibitory activity of heated RA / α-amylase inhibitory activity of non-heated RA) × 100.
The effect of RA concentration on its thermal stability was also evaluated by measuring its inhibitory activity against α-amylase from mealworms. After RA samples were dissolved in 1 mL distilled water to 0.1–10% (w/w) and stirred for 60 min at room temperature, each solution was divided into two test tubes. One test tube was boiled at 100 °C for 60 min, and the other was kept at room temperature. Both solutions were cooled in an ice bath and diluted to a final concentration of 0.05% (w/w). These suspensions were centrifuged at 10 000×g for 15 min at 4 °C, and the supernatants were diluted 25 times with distilled water and used as samples for the α-amylase inhibitory activity assay. Thermal stability was measured as the ratio of remaining α-amylase inhibitory activity of RA in the supernatant after heat treatment to that of non-heated RA at the same concentration.
Nitrogen solubility Nitrogen solubility was determined using the method described by Bera and Mukherjee (1989) with some modifications. After protein samples (200 mg) were suspended in 20 mL of 50 mM phosphate-citrate buffer (pH 3–8) and stirred for 60 min at room temperature, they were divided into two test tubes. One was heated to 80 °C for 20 min, and the other was kept at room temperature. Both tubes were centrifuged at 10 000×g for 15 min. Nitrogen content of the supernatants was determined by the Kjeldahl method. In addition, nitrogen content of total protein (100 mg) was measured without being dissolved in solution. Nitrogen solubility was calculated as follows: nitrogen solubility (%) = (nitrogen content of supernatant / nitrogen content of protein) × 100.
Emulsifying properties Emulsifying properties were determined according to the method of Pearce and Kinsella (1978) with some modifications. After protein samples (30 mg) were suspended in 3 mL of 50 mM phosphate-citrate buffer (pH 3–8) and stirred for 60 min at room temperature, the protein solutions were centrifuged at 10 000×g for 15 min. To form an emulsion, 0.8 mL corn oil and 2.4 mL of supernatant were shaken together and homogenized by a Polytron homogenizer (PT-1200E; Kinematica, Lucerne, Switzerland) at 25 000 rpm for 1 min at 20 °C. Then, 50 mL of emulsion was taken from the bottom of the test tube immediately or 10 min after homogenization and diluted with 5 mL of 0.1% SDS solution. The absorbance of the diluted emulsion was determined at 500 nm. Emulsifying activity (EA) was expressed as the absorbance at 500 nm measured immediately, and emulsifying stability (ES) was expressed as the absorbance measured 10 min after homogenization.
Foaming properties Foaming properties were determined using a modified method of Wang et al. (1999). After protein samples (24 mg) were suspended in 12 mL of 50 mM phosphate-citrate buffer (pH 3–8) and stirred for 60 min at room temperature, protein solutions were centrifuged at 10 000×g for 15 min. Protein solutions were then added to a 250 mL glass measuring cylinder. Nitrogen gas was flowed into the solution at 90 cm3/min for 15 sec followed by a rest of 15 sec, this procedure was repeated 10 times. Foam volume was measured immediately and 10 min after stopping the gas flow. The foaming capacity (FC) of proteins was expressed as the foam volume measured immediately after stopping the gas flow, and foaming stability (FS) was measured 10 min after stopping the gas flow.
Surface hydrophobicity Surface hydrophobicity was determined using a modified method of Iwami et al. (1986). Protein samples were suspended in 1 mL of 50 mM phosphate-citrate buffer (pH 3–8) and stirred for 60 min at room temperature. Protein solutions were centrifuged at 10 000×g for 15 min. Supernatants were diluted with 50 mM phosphate-citrate buffer (pH 3–8) to obtain protein concentrations ranging from 0.1 to 0.0125% (w/v). Then, 1 µL of 8.0 mM 1-anilino-8-naphthalenesulfonate in 100 mM phosphate buffer solution (pH 7.0) was added to 200 µL of each protein solution. Fluorescence intensity was measured with a spectrofluorometer (Synergy 2; BioTek, Tokyo, Japan) using excitation and emission wavelengths of 390 and 470 nm, respectively. Coefficient of linear regression analysis of fluorescence intensity vs. protein concentration (%) was used as an index of protein surface hydrophobicity.
Statistical analysis Data are expressed as mean ± standard deviation (SD). Significant differences between groups (p < 0.05) were determined using Tukey-Kramer tests.
Thermal properties The denaturation-endothermic peak of RA without the addition of reducing agent was initiated at 73.3 °C (Ti) and terminated at 114.8 °C (Tf), and peak temperature (Tp) and ΔH were determined as 100.8 °C and 3.15 J/g, respectively (Fig. 1A). By contrast, the Ti and Tf of the denaturation-endothermic peak of RA with DTT addition were 37.3 °C and 59.3 °C, and Tp and ΔH were 52.0 °C and 0.78 J/g, respectively. Furthermore, white turbidity was observed after the addition of DTT (Fig. 1B).
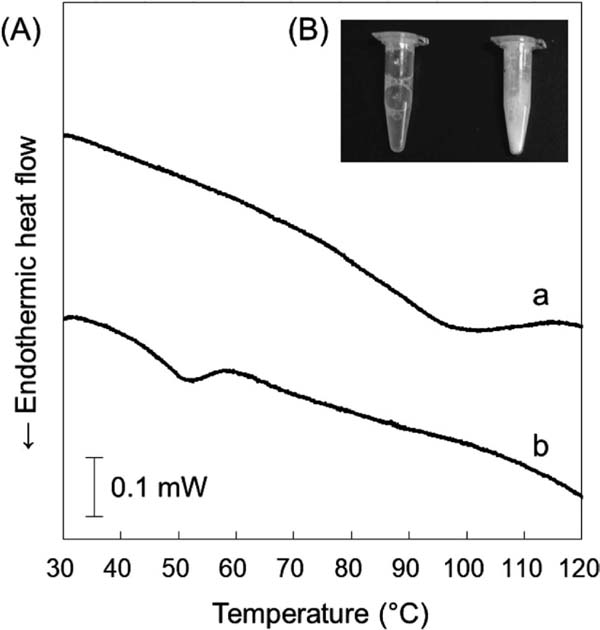
Changes in thermal sensitivity of rice albumin (RA) after the addition of a reducing agent.
A: DSC thermograms of RA untreated (a) or treated (b) with DTT for 16 hr at room temperature. B: Changes in the turbidity of RA after the addition of a reducing agent, left: untreated, right: treated with DTT.
As RA inhibits α-amylase activity from insects, its thermal stability was evaluated using mealworm α-amylase inhibitory activity as an index. The α-amylase inhibitory activity gradually decreased but remained at 50% even after heating to 100 °C for 120 min at an RA concentration of 0.1% (Fig. 2). Surface hydrophobicity gradually increased in inverse proportion to α-amylase inhibitory activity as heating time was prolonged. The thermal stability of RA at different concentrations remained after being heated to 100 °C for 60 min (Fig. 3). Also, α-amylase inhibitory activity decreased in a concentration-dependent manner, and the degree of heat-induced aggregation increased in a concentration-dependent manner (data not shown).
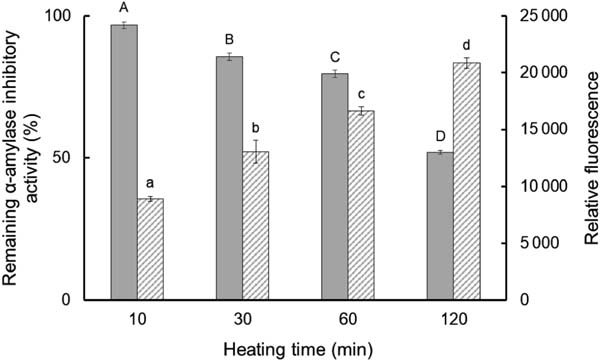
Remaining α-amylase inhibitory activity and surface hydrophobicity of rice albumin (RA) with different heating times.
Solid bar: α-amylase inhibitory activity, hatched bar: surface hydrophobicity. Heating temperature was 100 °C. Values with different letters are significantly different at p < 0.05. Each value is the mean of three experiments with SD shown as a vertical bar.
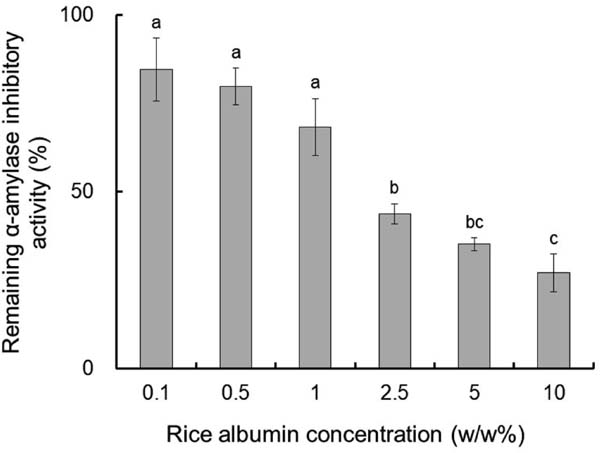
Remaining α-amylase inhibitory activity of rice albumin (RA) at different concentrations after heating at 100 °C for 60 min.
Values with different letters are significantly different at p < 0.05. Each value is the mean of three experiments with SD shown as a vertical bar.
Nitrogen solubility The nitrogen solubility of protein samples dissolved in solutions of various pH levels was measured before and after heating to 80 °C for 20 min (Fig. 4). RA exhibited high solubility at every pH measured, even after heating. The solubility of DEW was high before heating but drastically decreased after heating. Although casein hardly dissolved in a solution with a pH lower than 5, it dissolved in a solution of pH higher than 7 under both non-heated and heated conditions. The solubility of SPI was not as high as that of the other protein samples, but it gradually increased with increasing pH under both non-heated and heated conditions.
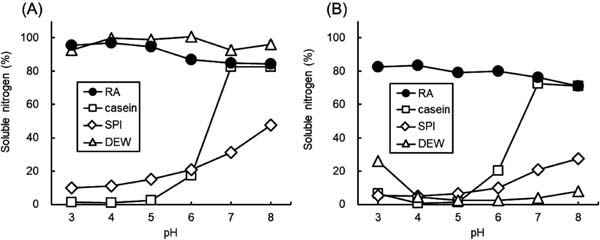
Nitrogen solubility profiles of rice albumin (RA), casein, soy protein isolate (SPI), and dried egg white (DEW) at different pH values.
A: non-heat-treated, B: heat-treated (80 °C, 20 min). Each value is the mean of three experiments with SD shown as a vertical bar.
Emulsifying properties Casein and SPI showed low EA values at pH ≤ 5 (Fig. 5). The EA of RA was stable and retained a high value at every pH. All proteins showed high EA around neutral pH levels (pH 6–8), consistent with a previous report.12 Different from casein, SPI, and DEW, the ES of RA was relatively high at acidic pH but low at pH 6. The ES of SPI and DEW were low at every pH measured, whereas that of casein gradually increased as pH increased and had almost the same value as that of RA at pH 7–8.
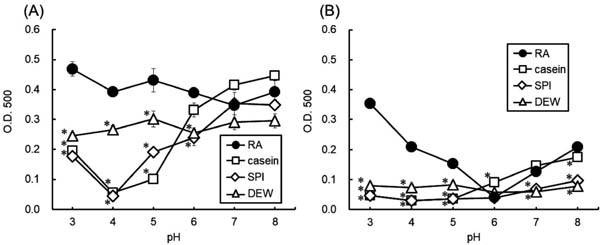
Emulsion activity and stability of rice albumin (RA), casein, soy protein isolate (SPI), and dried egg white (DEW) at different pH values.
A: emulsion activity, B: emulsion stability after 10 min. Values with an asterisk are significantly different from the corresponding RA value at p < 0.05. Each value is the mean of four experiments with SD shown as a vertical bar.
Foaming properties RA and DEW showed stable FC at all pH levels, although casein and SPI showed low FC values at pH ≤ 5 (Fig. 6). At an acidic pH (pH 3–5), the FCs of RA and DEW were higher than those of casein and SPI. At a neutral pH (pH 6–8), the FC of RA was almost the same as those of SPI and DEW but lower than that of casein. Foam volume of all proteins decreased after 10 min. The FS of RA was slightly high at pH 4–5 but lower above pH 6.
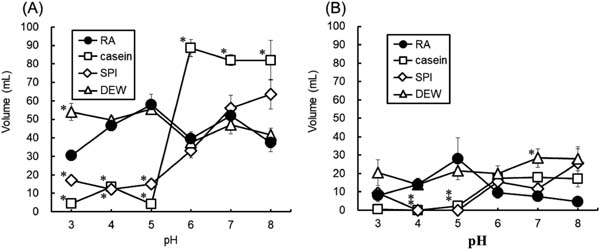
Foaming capacity and stability of rice albumin (RA), casein, soy protein isolate (SPI), and dried egg white (DEW) at different pH values.
A: foaming capacity, B: foaming stability after 10 min. Values with an asterisk are significantly different from the corresponding RA value at p < 0.05. Each value is the mean of four experiments with SD shown as a vertical bar.
Surface hydrophobicity The surface hydrophobicity of RA and DEW was relatively stable and high at every pH, whereas that of SPI and DEW was low at pH 4–5 (Fig. 7).
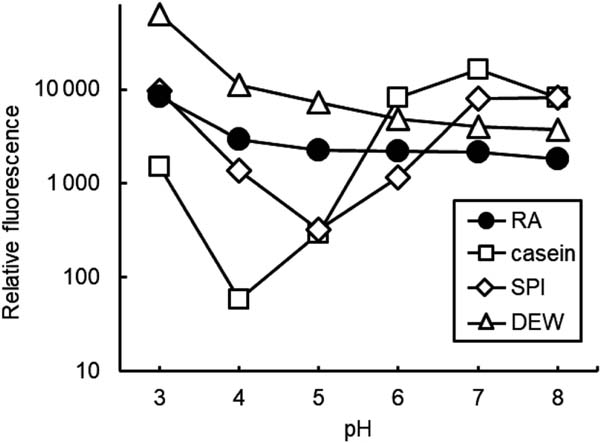
Surface hydrophobicity profiles of rice albumin (RA), casein, soy protein isolate (SPI), and dried egg white (DEW) at different pH values.
To add proteins to various foods as a healthful food material, not only their physiological functions but also their physicochemical functions, such as solubility and surface properties, are of great importance. In addition, their functions must be maintained after thermal sterilization, which is an indispensable process in the food industry, and in low pH conditions, as the pH of some foods is between 2 and 5. However, protein functions are often affected by heating and changes in pH.
RA is a promising functional protein for preventing diabetes because it suppresses elevations in blood glucose level upon starch or glucose loading, as shown in our preceding study (Ina et al., 2016). RA is a 16-kDa protein that is hydrolysed to 14 kDa and small peptides by digestive enzymes. However, the high molecular weight peptide of 14 kDa is not further hydrolysed even after treatment by pepsin for 2 hr and pancreatin for 6 hr (Ina et al., 2016). This high indigestibility may be attributed to its tight structure supported by five intramolecular disulphide bonds, determined by LC-MS/MS and structural analyses. The suppressive effect of RA on hyperglycaemia might be due to adsorption of glucose onto indigestible high molecular weight peptides of 14 kDa, like dietary fibre (Ina et al., 2016). As these intramolecular disulphide bonds stabilize the protein structure and make it resistant to denaturation, we examined the thermal and acid stability of RA for its application to various types of foods.
The thermal properties of RA were evaluated by DSC, α-amylase inhibitory activity, and surface hydrophobicity. The contribution of disulphide bonds to the thermal stability of proteins was assessed by measuring the denaturation temperature of RA with or without the addition of a reducing agent using DSC. The effects of heating time and RA concentration on denaturation were evaluated by measuring inhibitory activity against mealworm α-amylase and surface hydrophobicity.
RA exhibited excellent thermal stability as a result of its disulphide bonds. DSC analysis revealed that the peak temperature (Tp) of RA (100.8 °C) was much higher than that of DTT-treated RA (52.0 °C), suggesting that the disulphide bonds stabilize the conformational structure of RA. The decrease in ΔH of RA from 3.15 J/g to 0.78 J/g with the addition of DTT, and the observation of white turbidity after DTT addition also indicate the importance of disulphide bonds to structural stability. This decrease in ΔH likely occurred because RA denatured and aggregated in the presence of DTT before DSC analysis. Thermal stability of RA was further evaluated by measuring the α-amylase inhibitory activity remaining after heating and the degree of surface hydrophobicity. The α-amylase inhibitory activity remained at almost 80% after heating to 100 °C for 60 min and 50% even after heating for 120 min at an RA concentration of 0.1%. The degree of surface hydrophobicity of RA increased in a time-dependent manner, indicating that the conformation of RA irreversibly changed after heating. This result is consistent with the DSC analysis, since the heating temperature for the measurement of thermal stability was almost the same as the Tp of RA (100.8 °C) observed by DSC analysis. The inhibitory activity of RA against α-amylase decreased as the RA concentration increased. This might be because an increase in RA concentration increased its interactions, promoting the aggregation of RA.
Casein, ovomucoid, and T4 lysozyme are well-known heat-resistant proteins. Casein is rich in proline and has very few intramolecular disulphide bonds. This structural feature limits it to a tertiary structure, contributing to its thermal stability. On the other hand, ovomucoid (Matsuda et al., 1982) and T4 lysozyme (Wetzel et al., 1988) have multiple disulphide bonds, which has a crucial role in structural stability. In addition, wheat albumin, which has high homology with RA, is reported to be heat-resistant due to its intramolecular disulphide bonds (Silano and Zahnley, 1978; Oneda et al., 2004). These previous findings support our observation that disulphide bonds contribute to the thermal stability of RA.
The observed thermal stability of RA led us to investigate the acid stability of RA, as the disulphide bonds stabilizing the protein structure are hardly cleaved under acidic conditions. Nitrogen solubility, emulsifying and foaming properties, and surface hydrophobicity of RA between pH 3 and 8 were comparable to those of commonly used and well-investigated food proteins such as casein, SPI, and DEW.
The degree of nitrogen solubility in this study was measured by the ratio of the amount of protein remaining in the supernatant to total protein at different pH levels and after various centrifugations. The observed patterns of nitrogen solubility of casein, SPI, and DEW were in good agreement with those reported in the literature (Ahmedna et al., 1999). The nitrogen solubility of casein was almost 0 at pH 3–5, probably due to its isoelectric point (pI 3–5), but was soluble at pH 7–8. The nitrogen solubility of SPI was not as high, even at neutral pH, in accord with the common understanding that SPI is a poorly soluble protein. The nitrogen solubility of DEW was high at pH 3–8 before heating, but was hardly soluble at any pH after heating to 80 °C for 20 min. On the other hand, the nitrogen solubility of RA was significantly higher than that of casein and SPI, and was more than 80% at all pH levels, similar to DEW, before heating. In addition, nitrogen solubility remained at around 80% even after heating. As RA heated to 100 °C maintained inhibitory activity against α-amylase, its tertiary structure would be maintained under acidic conditions even after heating, and ionizable amino and carboxyl groups would remain on the surface of RA to form strong hydrogen bonds with water. Thus, as a highly soluble protein, RA could be used in various liquid or reconstituted foods and beverages. Furthermore, RA scarcely produces an insoluble fraction during processing that would reduce the quality of products, especially in terms of appearance. This feature of RA could contribute to a reduction in waste during processing, in addition to circumventing the need for a separation process.
Emulsifying and foaming properties are important for evaluating water-soluble proteins for use as food additives. The emulsifying and foaming properties of RA are expected to be greater than those of other proteins because these aqueous physicochemical properties are strongly affected by solubility (Kinsella and Melachouris, 1976; Ahmedna et al., 1999), and RA is soluble in solutions of various pH (pH 3–8) even after heating. The observed EAs of casein, SPI, and DEW in this study were consistent with those previously reported (Chobert et al., 1988; Tomotake et al., 2002; Chen et al., 2012). The EA and stability of RA at acidic pH were significantly higher than those of other proteins. The low EA and stability of casein and SPI were likely due to their low solubility at acidic pH. The EA of DEW was about two-thirds that of RA and not as low as those of casein and SPI, probably because the solubility of DEW was high even at acidic pH. Although the emulsion stability of RA was higher than that of other proteins at acidic pH, it was low at pH 6. This might be because the isoelectric point of RA is between 6 and 7 (Matsuda et al., 1991; Ina et al., 2016), and a low net charge might have made emulsions unstable. For all proteins, their foaming capacities showed similar patterns as their nitrogen solubilities. The foam volume of RA was almost the same as that of DEW at all pH levels and was higher than that of casein and SPI at pH 4 and 5, which was similar to the observed trend in nitrogen solubility. As protein solubility requires high net charges that influence the adsorption of proteins at the air-water interface (Cherry and McWatters, 1981), high protein solubility is thought to improve FC (Townsend and Nakai, 1983). FS of the proteins determined in this study seemed quite low; however, similar results were previously reported. Ahmedna et al. (1999) evaluated FS by measuring the liquid volume remaining 30 min after foaming SPI, DEW and sodium caseinate solutions. They reported that approximately 10% of the foam remained as to these proteins. In addition, Wang et al. (1999) evaluated the foam stability of rice bran protein at pH 7.4, and found that the foam volume remaining after 30 minutes decreased to 30% of the initial volume. Proteins susceptible to denaturation would be easily aligned on the surface of bubbles and stabilize the foam. The reason for the low FS of RA might be because of its high resistance to denaturation. This assumption was supported by the stable surface hydrophobicity of RA at every pH. Although FS of RA was not so high, RA showed better functional properties than other proteins at acidic pH, demonstrating that RA could be used as an emulsifying and foaming agent.
RA showed excellent thermal and acid stability, likely due to its robust structure supported by disulphide bonds. This information on thermal properties is useful for processing strategies and heat-processing design. In addition, we demonstrated that RA is an excellent emulsifying and foaming agent that can be used at various pH levels. Therefore, RA could be used as a functional protein to prevent postprandial hyperglycaemia with optimal processing properties, such as solubility and emulsifying and foaming properties, in various foods. It is important to note that indigestible proteins with a rigid structure due to disulphide bonds like rice protein may act as an allergen (Matsuda et al., 1991), although rice does not induce severe allergic symptoms compared to other grains such as wheat and buckwheat. Further study of the safety of RA is necessary to demonstrate that RA is not only useful but also acceptable to many people.