Abstract
Four extraction techniques including enzyme-assisted supercritical carbon dioxide extraction (ESC-CO2), supercritical carbon dioxide extraction (SC-CO2), ultrasound-assisted extraction (UAE) and Soxhlet extraction (SE) were evaluated to efficiently extract the oil from Decaisnea insignis seeds in this study. Lipid profiling by GC-MS confirmed seven fatty acids and six phytosterols in all oils obtained, in which palmitoleic acid (from 475.4 ± 12.0 by SE to 604.9 ± 26.1 mg/g oil by ESC-CO2) and β-sitosterol (from 35.6 ± 1.2 by SE to 60.9 ± 2.3 mg cholestanol equivalent/100 g oil by ESC-CO2) predominated, respectively. Furthermore, the oil yielded by ESC-CO2 displayed higher polyphenol content and stronger DPPH and ABTS radical scavenging capacities. These results suggested that D. insignis seed oil is considered an alternative source of palmitoleic acid, and ESC-CO2 could be explored as an environmentally friendly technology for extracting plant oil.
Introduction
Decaisnea insignis (Griffith) Hook. f. & Thomson, also known as Decaisnea fargesii Franch, is the only species in genus Decaisnea of Lardizabalaceae and native to eastern Asia from central to south-western China, extending into Bhutan, Myanmar, Nepal, Sikkim and north-eastern India (Li et al., 2017). The plant has diverse economic benefits because each organ of this plant plays a useful role in human's life. The root of D. insignis has been frequently used as a heat-clearing and detoxicating herb in Traditional Chinese Medicine (TCM) for centuries (Wang et al., 2018a), which was recently found to contain cytotoxic triterpenoid glycosides and show anti-oxidative and anti-inflammatory activities in vitro (Jin et al., 2014). Moreover, still experiencing as a wild and wonderful indigenous fruit in China, the flesh of the ripe fruit is rich in organic acids, polyphenols, as well as macro- and micro-nutrients.
Furthermore, the plant seed oil has attracted considerable interest in recent years due to its biodiesel potential and nutritional value. The plant seed makes up approximately 16% of the total weight of the fruit and was found to be an ideal candidate for biodiesel production (Qin et al., 2015). More interestingly, D. insignis seed oil is characterized by a high proportion of beneficial unsaturated fatty acids such as palmitoleic, oleic and linoleic acids, with a particularly high content of the first one (∼ 60%) (Liu and Liu, 2011; Sun et al., 2012a). Palmitoleic acid, otherwise known as omega-7 fatty acid, has a low melting point (0.5–1 °C) and good oxidative stability, making it a suitable ingredient for enriched or fortified foods (Gao et al., 2017). On the other hand, increasing evidence has demonstrated that this fatty acid has beneficial effects on insulin sensitivity, cholesterol metabolism, and hemostasis (de Souza et al., 2018; Frigolet and Gutierrez-Aguilar, 2017; Yang et al., 2011). However, nature's most concentrated sources of palmitoleic acid are reported for only a few plants like Macadamia nuts (30%) and sea buckthorn pulp (34%) (Dubois et al., 2007), and the potential of D. insignis seed as an alternative source of palmitoleic acid has long been underestimated. Therefore, the oil recovery from D. insignis seed is a fundamental step to exploit the potential of this plant oil.
Traditionally, conventional Soxhlet and heated reflux solvent extraction techniques have been widely used to extract oils from plants of interest. Unfortunately, these solvent-based extraction methods often suffer from time- and solvent-consumption, low yield, and possible contamination of the final products. Consequently, several cost-effective and clean alternatives, such as ultrasound-assisted extraction (UAE), microwave-assisted extraction (MAE), and supercritical fluid extraction (SFE) have been developed in the past few decades (Chemat et al., 2017). Among these SFE involves the use of gases, usually supercritical carbon dioxide for extraction process. It appears to be the most promising technology for lipid or essential oil recovery due to its better retention of aromatic or thermally unstable compounds, lack of harmful solvent residue and higher yield (Da Porto et al., 2016; Sahena et al., 2009). Meanwhile, enzymatic pretreatment of plant materials may enable better release and higher recovery of bioactive compounds, and has been increasingly applied either alone or in combination with these innovative extraction technologies (Chen et al., 2016; Liu et al., 2016). For instance, Ezeh et al. (2016) reported that the mixture of protease, α-amylase, and viscozyme increased the recovery of tiger nut (Cyperus esculentus) oil during mechanical pressing.
As mentioned above, D. insignis seed oil studied previously was yielded by Soxhlet extraction (SE) or mechanical pressing. However, the information about the effect of advanced extraction techniques on its fatty acid profile and quality is still lacking. Meanwhile, to the best of our knowledge, other valuable compounds such as phytosterols, tocopherols, and polyphenols in D. insignis seed oil have not yet been reported. This study therefore set out to evaluate the oil yield, physicochemical properties, chemical profile and antioxidant capacity of D. insignis seed oils obtained by four different extraction methods including enzyme-assisted supercritical carbon dioxide extraction (ESC-CO2), supercritical carbon dioxide extraction (SC-CO2), UAE and SE.
Materials and Methods
Seeds The fruits of D. insignis were harvested in their maturity between September and October of 2016, in Zhengping County, Shaanxi Province, China. The fruits collected were locally shelled by hand, and then the resulting seeds were shipped to our laboratory, dried at 50 °C to constant weight, ground with a particle size less than 500 µm, and stored at −4 °C till analysis.
Chemicals Supelco® 37 Component FAME Mix (CRM47885), Folin-Ciocâlteu's reagent, 2,2-diphenyl-1-picrylhydrazyl (DPPH·), 2,2-azinobis-(3-ethylbenzothiazoline)-6-sulfonic acid (ABTS), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), cholestanol, rutin, gallic acid α-, β-, γ- and δ-tocopherols were purchased from Sigma-Aldrich (USA). Trimethylsilyl chloride (TMCS) and N,O-bis(trimethylsilyl) trifluoroacetamide (BSTFA) were supplied by Adamas Reagent Co. Ltd. (China). Alcalase (1 500 U/g) and pectinase (1 500 U/g) were obtained from Jingbo Bio-Technology Ltd. (China). Chromatographic grade hexane was purchased from Alltech Scientific Co. Ltd. (China). All other chemicals and reagents used in this study were of analytical grade if not specified otherwise.
Oil extraction The Soxhlet extraction (SE) was accomplished based on the previous method optimized by Sun et al. (2012b). Using a Soxhlet apparatus, ground dried seeds (10.0 g) were extracted with 200 mL of n-hexane for 8 h at 77 °C. The lipid extracts were then evaporated under reduced pressure at 40 °C and the resulting oil was stored at −20 °C for further analysis. The crude oil yield was measured gravimetrically and reported as follows:
Where Y is the crude extraction yield (%), moil and msample are the oil crude extract mass (g) and the extracted dried seed mass (g), respectively.
The applied ultrasound-assisted extraction (UAE) methodology followed a previously established procedure (da Silva et al., 2017) with some modifications. Ground dried seeds (10.0 g) were macerated with 100 mL of n-hexane and the mixture was then extracted at 40 °C for 60 min in a continuous ultra-sonication (SB25-12DTD, Ningbo Scientz Biotechnology Co., Ltd., China), with outpower of 300 W and irradiation frequency of 40 KHz. After centrifugation of the mixture at 5 000 rpm for 5 min, the supernatant was processed similarly as in the SE above.
The supercritical carbon dioxide extraction (SC-CO2) was carried out on a supercritical fluid extractor Spe-ed SFE-2 (Applied Separation, USA) (Millao and Uquiche, 2016). Briefly, ground dried seeds (20.0 g) were loaded into a 100 mL extraction vessel and the extraction conditions were set at 30 Mpa, 40 °C, dynamic extraction with gaseous carbon dioxide of 0.2 g/min flow for 3 h, respectively. The extracted oil was collected in glass bottles.
In the ESC-CO2, alcalase and pectinase were selected to pretreat the sample powder according to You et al. (2011). In brief, the optimal concentration of each enzyme (alcalase : pectinase = 1:1, m:m) was selected in preliminary experiments by testing the impact of different concentrations of the enzymes on the oil yield at pH 5.0 and 50 °C for 60 min (data not shown). Ground seed powder (20.0 g) was mixed with 40 mL of 0.1 M citrate phosphate buffer solution including the optimal enzyme cocktail (3 000 U/mL) and incubated at 50 °C for 1.5 h. The mixture then was centrifuged at 5 000 rpm for 10 min, and the enzymatic-hydrolyzed solid fraction was collected, dried at 50 °C to constant weight, and further extracted using the SC-CO2 method applied above.
Physicochemical parameters Quality properties such as acid, peroxide, iodine and saponification values of the D. insignis seed oils obtained were determined according to AOAC (2000).
Fatty acid profile analysis Fatty acid profile was tested by GC-MS after derivatization to fatty acid methyl esters as described by Li et al. (2016). The fatty acid methyl esters were analyzed using GC-MS 2010 Ultra (Shimadzu, Kyoto, Japan) with a Rxi-5Sil MS Cap. column (30 m × 0.25 mm i.d, 0.25 µm film thickness, Risetech Technology Co., Ltd, Beijing, China). The fatty acid methyl esters (1 µL) were injected at 290 °C of the injection temperature and high purity helium was used as the carrier gas of 1.0 mL/min flow-rate. The temperature of the column was kept at 110 °C for 1 min, and increased at 5 °C per min to 205 °C where it was held for 8 min, and then increased at 5 °C per min to 250 °C and further increased at 8 °C per min to 310 °C where it remained for 2 min. Ion source and interface temperatures were 230 °C and 300 °C, respectively. Standard fatty acid methyl esters were used to quantify the fatty acid methyl esters in the oil samples.
Phytosterol analysis The GC-MS program for the sterol composition of the oils was followed as described in our previous report (Wang et al., 2018b). After saponification and derivatization, 1 µL of derivatives was injected for sterol analysis by the same column used in the fatty acid profile analysis. The phytosterol profile was determined by comparison with NIST 98 spectra library and the reported data, while the content of each phytosterol was quantified based on peak areas. The results were expressed as milligrams of cholestanol (internal standard) equivalent (CSE) per 100 g oil (mg CSE/100 g oil).
Extraction for polyphenolic compounds Polyphenolic compounds of D. insignis seed oil were extracted according to Slatnar et al. (2015). Ten grams of D. insignis seed oil was dissolved in 10 mL of n-hexane and 2.0 mL of CH3OH/H2O (v/v, 60:40) was added subsequently. After vortex-mixed for 2 min, the mixture was centrifuged at 3000 rpm for 5 min. The methanol fraction was collected and the oil sample was extracted twice later with 2 mL of methanol. The pooling supernatant was further washed by n-hexane and dried with a gentle stream of nitrogen. The resulting extracts were redissolved in 1 mL of methanol/water (50:50, v/v).
Tocopherol analysis Tocophenol content was determined according to the previously established method by Hussain et al. (2013) with some modifications. In brief, one gram of D. insignis seed oil was dissolved in 5 mL of n-hexane and filtered through a 0.45 µm PTFE membrane syringe filter, then approximately 0.5 mL of the clear solution was collected in the GC vial and stored at −20 °C until analysis on GC. GC-14A (Shamidzu Cooperation, Japan) equipped with Rxi®-5Sil MS column (fused silica, 30 m × 0.25 mm i.d, 0.25 µm film thickness) and flame ionization detector (FID), was used for tocopherol analysis. The temperature program was as follows: The temperature of column was set at 100 °C and then programmed at 15 °C per min to 280 °C and held for 15 min. The linear velocity flow mode was chosen. Split injection with a split ratio of 1:100 was used and injection temperature and detector temperature were 290 °C. High purity helium was used as the carrier gas of 1.0 mL/min flow rate. The peaks in the experimental samples were identified by comparing the retention times of tocopherol standards using Shimadzu GC Solution computer software.
Determination of total phenolic content Total phenolic content (TPC) was determined according to the Folin-Ciocâlteu's method described by Zhou et al. (2017). The results were expressed as mg of gallic acid equivalents (GAE) per 100 g of oil.
Determination of total flavonoid content Total flavonoid content (TFC) in D. insignis seed oil was measured according to the aluminum chloride colorimetric assay by Brahmi et al. (2012). The results were expressed as mg of rutin equivalents (RE)/100 g oil.
Antioxidant activity ABTS and DPPH radical scavenging activities of D. insignis seed oil were determined according to Fiorini et al. (2018). Trolox was used as the reference standard, and the results were expressed as µmol Trolox equivalents (TE) / g oil.
Statistical analysis All experimental data were expressed as means ± standard deviation (mean ± SD, n = 3). One-way analysis of variance (ANOVA) was performed with DPS statistical software (v7.5, China), and Tukey's test was used to determine significant differences between means. The differences were considered statistically significant at p < 0.05.
Results and Discussion
Oil yield and physicochemical properties The effect of four extraction technologies on the extraction yield and physicochemical properties of D. insignis seed oils was investigated and the results are presented in Table 1. Four extraction methods showed varied extraction efficiency with the crude oil yields ranging from 21.4 ± 0.4% to 30.6 ± 0.4%. SE and UAE had the most and the least extraction efficiency, respectively, whereas SC-CO2 and ESC-CO2 produced oil with the recovery from 81.1% to 89.8% of SE, respectively. Similarly, Belayneh et al. (2015) reported that the Camelina seed oil extracted by optimized SC-CO2 was 88% of that from the SE extraction. Most interestingly, ESC-CO2 provided a significantly higher extraction yield compared with SC-CO2 in the present study. This improvement could be attributed to the enzymatic pretreatment that partly hydrolyzed macromolecules such as carbohydrates and proteins and subsequently destructed cell wall, thereby facilitating the percolation of supercritical CO2 fluid and the release of lipid from the corresponding plant matrices. In fact, enzymatic pretreatment of oilseeds can also favor oil recovery using other extraction methods apart from SC-CO2. An oil yield increment of 106% over SE was observed in enzyme-assisted SE of the oil from grape seeds under optimum conditions (Passos et al., 2009). More recently, Liu et al. (2019) have reported that efficient ultrasonication extraction of oil from Sapindus mukorossi seed kernels was achieved after the seed kernels have been pre-incubated with different enzymes either alone or in mixture, especially an enzyme cocktail of cellulase, pectinase, and hemicellulase in equal proportion. It might be expected that the same enzymatic hydrolysis in ESC-CO2 can further increase the D. insignis seed oil yields of SE or UAE in this work.
Table 1.
Oil yield and physicochemical properties of
D. insignis seed oils extracted by four extraction methods
| Properties |
SE |
UAE |
SC-CO2 |
ESC-CO2 |
| Oil yields (%) |
30.6 ± 0.4a |
21.4 ± 0.4d |
24.8 ± 0.2c |
27.5 ± 0.2b |
| Peroxide value (mmol/kg) |
5.5 ± 0.1a |
4.5 ± 0.2b |
3.9 ± 0.2c |
3.9 ± 0.1c |
| Iodine value (g I2/100 g oil) |
89.0 ± 0.1a |
91.0 ± 0.2a |
91.8 ± 0.2a |
92.7 ± 0.3a |
| Saponified value (mg KOH/g oil) |
133.0 ± 0.6c |
132.1 ± 0.3c |
135.6 ± 0.6b |
137.1 ± 0.1a |
| Acid value (mg KOH /g oil) |
1.1 ± 0.2b |
1.1 ± 0.1b |
1.2 ± 0.2b |
1.2 ± 0.1a |
a, b, c, d values in the same line with different letters are significant difference at
p < 0.05.
Furthermore, the quality properties of D. insignis seed oils were also somewhat varied depending on the different extraction methods used (Table 1). The peroxide values of the oils extracted by SC-CO2 or ESC-CO2 were slightly lower than these of oils yielded by SE and UAE (p < 0.05), suggesting supercritical fluid extraction performed at a lower temperature can prevent many undesirable reactions such as oxidation, hydrolysis, degradation, and rearrangement, thus decrease the contents of free fatty acids, peroxides and hydroperoxides. The iodine values (89.0 ± 0.1 to 92.7 ± 0.3 g I2/100 g oil) of D. insignis seed oils obtained by four extraction methods were no significant difference (p > 0.05), and within the ranges previously reported. This suggested that D. insignis seed oil contained a larger amount of monounsaturated fatty acids, as evidenced in the following GC-MS analysis. Moreover, both saponified and acid values of ESC-CO2 extracted oil were a little higher than that of oils extracted by other three methods. One reason for this difference may be associated with the prolonged activity of some enzymes such as lipases native to the seeds and originated from alcalase and pectinase, since the optimum temperature and pH for lipases of various origins range between 30 and 80 °C, and between 4.5 and 11, respectively. In accordance with the present results, previous studies have also demonstrated that enzymatically extracted pumpkin oil and wild apricot oil contained more free fatty acids and displayed high acid values (Bisht et al., 2015; Konopka et al., 2016). Collectively, the physicochemical parameters of D. insignis seed oils obtained by four extraction methods were within the normal ranges of most vegetable oils, revealing their potential for food applications.
Fatty acid profiling and quantification Table 2 shows the fatty acid compositions of the oils obtained by four extraction techniques. Seven different fatty acids were identified by comparison with the retention times of thirty-seven fatty acid standards, and the fatty acid profiles of these oils were fairly the same despite the different extraction technologies used. Palmitoleic acid accounted for 62.6–64.7% of total fatty acid, a figure which markedly higher than the reported data. Currently, the highest reported content of palmitoleic acid in plants corresponds to the shrub sea buckthorn, whose pulp contains palmitoleic acid at 32–42% (Dubois et al., 2007). After palmitoleic acid, oleic acid and linoleic acid were also found to prevail in the obtained oils with the average contents from 93.6 ± 5.4 to 121.4 ± 3.8, and from 94.6 ± 7.3 to 114.5 ± 9.1 mg/g oil, respectively. The two most common saturated fatty acids such as palmitic acid and stearic acid, together with linolenic acid, showed an average concentrations of 24.3 ± 2.1 to 42.8 ± 2.0, 20.3 ± 2.4 to 35.7 ± 5.1, 25.9 ± 0.02 to 34.1 ± 1.9 mg/g oil, respectively, and myristic acid was found in minor amounts of 0.7 ± 0.1 to 1.0 ± 0.1 mg/g oil.
Table 2.
Fatty acid composition of
D. insignis seed oils obtained by four extraction methods.
|
RT
(min) |
The content of fatty acids (mg/g oil) (percentage%) |
|
SE |
UAE |
SC-CO2 |
ESC-CO2 |
| Fatty acids |
|
|
|
|
| C14:0 |
14.273 |
0.7 ± 0.1c (0.1%) |
1.0 ± 0.1a (0.1%) |
0.8 ± 0.04b (0.1%) |
0.9 ± 0.03a (0.1%) |
| C16:1 |
18.064 |
475.4 ± 12.0b (64.7%) |
573.8 ± 14.0a (64.5%) |
512.2 ± 12.2b (62.6%) |
604.9 ± 26.1a (63.4%) |
| C16:0 |
18.522 |
24.3 ± 2.1c (3.3%) |
34.6 ± 2.5b (3.9%) |
31.6 ± 4.7b (3.9%) |
42.8 ± 2.0a (4.5%) |
| C18:2 |
22.066 |
94.9 ± 7.3b (12.9%) |
112.3 ± 5.1a (12.6%) |
98.8 ± 3.4b (12.1%) |
114.5 ± 9.1a (12.0%) |
| C18:1 |
22.258 |
93.6 ± 5.4c (12.8%) |
107.9 ± 3.0b (12.1%) |
116.0 ± 4.6a (14.2%) |
121.4 ± 3.8a (12.7%) |
| C18:0 |
22.99 |
20.3 ± 2.4b (2.8%) |
29.5 ± 3.4a (3.3%) |
28.7 ± 4.4a (3.5%) |
35.7 ± 5.1a (3.7%) |
| C18:3 |
29.099 |
25.9 ± 0.02b (3.5%) |
29.8 ± 1.8ab (3.4%) |
29.7 ± 4.0ab (3.6%) |
34.1 ± 1.9a (3.6%) |
| Fatty acid groups |
|
|
|
|
| SFA |
|
45.2 ± 4.5c (6.4%) |
65.1 ± 5.9b (7.3%) |
61.1 ± 9.1b (7.5%) |
79.5 ± 70.2a (8.3%) |
| MUFA |
|
569.1 ± 6.6d (77.4%) |
681.7 ± 11.0b (76.7%) |
628.2 ± 7.6c (76.8%) |
726.3 ± 22.3a (76.1%) |
| PUFA |
|
120.7 ± 7.3c (16.4%) |
142.1 ± 6.9a (16.0%) |
128.5 ± 0.6b (15.7%) |
148.5 ± 7.2a (15.6%) |
| TFA |
|
735.0 ± 5.2d |
889.0 ± 10.0b |
817.8 ± 16.1c |
954.3 ± 36.7a |
| UFA/TFA% |
93.4 ± 0.1a |
92.7 ± 0.6ab |
92.5 ± 1.0ab |
91.7 ± 0.4b |
a, b, c, d values in the same line with different letters are significant difference at
p < 0.05.
RT: the retention time in GC-MS, TFA: Total fatty acid, SFA: Saturated fatty acid, MUFA: Monounsaturated fatty acid, PUFA: Polyunsaturated fatty acid.
Moreover, the proportion of unsaturated fatty acids of D. insignis seed oils was up to 90%, which was significantly higher than soybean oil (78%), olive oil (81%), and sunflower oil (87%) (Nehdi et al., 2018). It was noted that the monounsaturated fatty acids were responsible for nearly 84% of unsaturated fatty acids in D. insignis seed oils. Regarding the extraction methods used, the total fatty acid content obtained by ESC-CO2 accounted for the highest proportion of crude oil yield (95.4%), followed by UAE (88.9%), SC-CO2 (81.8%) and SE (73.5%). It might be explained that the combination of enzymatic hydrolysis and SC-CO2 was more selective in extracting lipid from the D. insignis seeds than UAE and SE. Meanwhile, too many impurities may be dissolved in the SE extracted oils by a prolonged extraction (8 h), leading to the lower fatty acid content. In fact, crude plant oils contain amounts of undesirable compounds such as free fatty acids, waxes, polar lipids, oxidation products, metal ions and pigments, which necessitate refining (Vaisali et al., 2015).
Phytosterol profiling and quantification Phytosterol composition of D. insignis seed oils under four extraction techniques was also characterized using GC-MS analysis. Six phytosterols including campesterol, stigmasterol, 24-methylcholesterol, β-sitosterol, lanosterol, and Δ9,24-lanosterol were identified in the obtained oils by the detection of the parent molecular ions and the fragmentation patterns of corresponding trimethylsilyl derivatives (Table 3, Fig. 1).
Table 3.
Relative retention times and fragmentation ions used in identification of
D. insignis seed oil phytosterols.
| Peaks |
Compounds |
RT (min) |
M+ (m/z) |
Main fragmentation ions, m/z (RI) |
References |
| 1 |
Campestreol |
15.86 |
472 (21) |
382 (43), 343 (56), 315 (5), 255 (13), 213 (11) 145 (26), 129 (100), 73 (69) |
Yang et al., 2009 |
| 2 |
Stigmasterol |
16.38 |
484 (20) |
394 (21), 379 (10), 282 (2), 213 (14), 173 (12), 129 (60), 95 (19), 83 (100) |
Cunha et al., 2006 |
| 3 |
24-Methylcholesterol |
17.39 |
472 (13) |
457 (7), 382 (57), 367 (32) |
Suzuki et al., 1995 |
| 4 |
β-Sitosterol |
17.80 |
486 (28) |
397 (25), 396 (78), 381 (26), 357 (66), 129 (98), 95 (54), 73 (82) |
Yang et al., 2009 |
| 5 |
Lanosterol |
18.58 |
498 (16) |
483 (26), 393 (82), 311 (8), 283 (7), 109 (48), 69 (98) |
Hammann & Vetter, 2016 |
| 6 |
Δ9,24-Lanosterola |
19.61 |
498 (12) |
483 (15), 393 (50), 241 (10), 109 (35), 95 (40), 81 (28), 69 (100) |
|
a Means tentatively identified by GC/MS data.
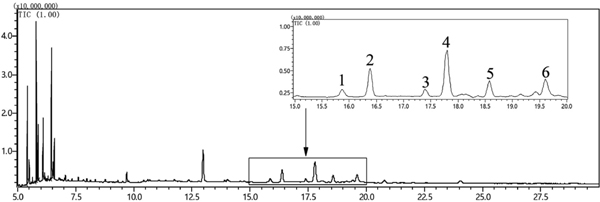
The phytosterol concentrations in D. insignis seed oils on cholesterol basis are reported in Table 4. The total content of the analyzed phytosterols ranged from 145.3 ± 6.2 to 205.1 ± 10.5 mg CSE/100 g oil, and the content of individual phytosterols of the ESC-CO2 extracted oil was, however, higher than that of oils obtained by other three extraction methods. These results indicated that ESC-CO2 was a more efficient method for extracting phytosterols from D. insignis seeds. Phytosterols are the characteristic structural components of plant cell membranes and hence a destruction of cell membranes is required to extract these compounds. As in this study, ESC-CO2 and UAE appeared to have a greater destructive effect on plant cells due to enzymatic hydrolysis or ultrasound cavitation, resulting in the release of a large amount of phytosterols. Similarly, several previous studies also suggested that the amount of total phytosterol or individual phytosterols in the extract obtained by SC-CO2 extraction were both higher than in the conventional organic solvent extracts (Hrabovski et al., 2012; Uddin et al., 2015).
Table 4.
Phytosterol composition of
D. insignis seed oils obtained by four extraction methods (mg CSE /100 g oil).
| Compounds |
SE |
UAE |
SC-CO2 |
ESC-CO2 |
| Campestreol |
26.1 ± 0.9a |
20.0 ± 1.9b |
19.4 ± 1.0b |
22.2 ± 1.3b |
| Stigmasterol |
28.9 ± 0.7b |
31.0 ± 4.0b |
31.2 ± 1.5b |
40.0 ± 2.6a |
| 24-Methylcholesterol |
16.4 ± 1.1b |
13.5 ± 0.4c |
12.7 ± 1.8c |
19.8 ± 1.2a |
| β-Sitosterol |
35.6 ± 1.2c |
50.5 ± 3.4b |
36.7 ± 2.9c |
60.9 ± 2.3a |
| Lanosterol |
21.1 ± 0.7b |
24.1 ± 1.8a |
19.8 ± 1.4b |
27.2 ± 1.8a |
| Δ9,24-Lanosterol |
20.8 ± 1.0c |
26.0 ± 1.9b |
25.6 ± 1.0b |
35.0 ± 1.5a |
| Total sterol |
148.1 ± 2.1b |
165.2 ± 12.6b |
145.3 ± 6.2b |
205.1 ± 10.5a |
a, b, c, d values in the same line with different letters are significant difference at
p < 0.05.
Among these analyzed phytosterols, β-sitosterol was the predominant one in four different extracted oils, contributing to 24.0–30.6% of the total phytosterol content. β-Sitosterol is reported to display a wide spectrum of biological activities, such as anti-hypercholesterolemia, anti-hyperlipoproteinemic and anti-inflammatory activities (Choudhary and Tran, 2011). The second principal phytosterol was stigmasterol, whose percentage varied from 18.8 to 21.5%. Other than β-sitosterol and stigmasterol, the content of campesterol, lanosterol and Δ9,24-lanosterol only accounted for 10.8–17.6%, 13.3–14.6% and 14.1–17.6% of the total phytosterol content, respectively. To the best of our knowledge, there are no published reports describing phytosterols in D. insignis seed oil.
Tocopherol contents Tocopherols are a group of fat soluble antioxidants naturally occurring in plant oils. Four tocopherols including α-, β-, γ- and δ-tocopherols are more common in nature, with α-tocopherol being the most abundant (Schwartz, et al. 2008). As shown in Table 5, only α-tocopherol and γ-tocopherol were detected in D. insignis seed oils, and α-tocopherol was represented 79.7–86.1% of the total tocopherols in the oils obtained by four extraction methods in this work.
Table 5.
Composition of tocopherol of
D. insignis seed oils obtained by four extraction methods (mg /g oil).
| Compounds |
SE |
UAE |
SC-CO2 |
ESC-CO2 |
| α-Tocopherol |
4.8 ± 0.2a |
1.2 ± 0.01b |
5.1 ± 0.4a |
3.9 ± 0.9a |
| ɤ-Tocopherol |
0.7 ± 0.01b |
0.2 ± 0.01c |
0.8 ± 0.1a |
0.6 ± 0.01b |
| Total tocopherol |
5.6 ± 0.2a |
1.5 ± 0.01b |
6.1 ± 0.5a |
4.1 ± 0.8a |
a, b, c, d values in the same line with different letters are significant difference at
p < 0.05.
TPC, TFC, and antioxidant activities Polyphenols inherited in plant oils can effectively inhibit lipid oxidation reaction and enhance the stability of the oil products. As shown in Fig. 2, both TPC and TFC in D. insignis seed oils varied significantly among four extraction methods. The oil containing the highest level of TPC and TFC was yielded by ESC-CO2, and followed by UAE, SC-CO2, and SE, respectively. This difference can be explained that the combined enzymatic hydrolysis and SC-CO2 promote the release of polyphenols and result in the highest TPC and TFC in ESC-CO2 extracted oil, whereas the non-polar solvent n-hexane used in SE has strong affinity and solubility for lipid compounds rather than for polar ones like most of polyphenols and flavonoids. In addition, ultrasound is also destructive to seed cells, thereby leading to a higher level of TPC and TFC compared with SE and SC-CO2.

The antioxidant potential of the obtained oils was further assessed by DPPH and ABTS assays (Fig. 3). The capacity of the extracted oils to scavenge ABTS radicals was decreased in the order of ESC-CO2 > SC-CO2 > UAE > SE, whereas ESC-CO2 extracted oil also displayed stronger scavenging activity against DPPH radicals (13.3 ± 0.6 µmol TE/g oil) compared with other three extraction methods. It appears that ESC-CO2 offered better access to the antioxidants in D. insignis seeds of which content of TPC and TFC were positively related to the antioxidant activity observed. However, the antioxidant potential of D. insignis seed oil may be underestimated in this work because the polyphenolic extracts tested were extracted by the mixture of methanol and water, in which lipophilic components such as tocopherols and fatty acids generally have a relatively lower solubility. Such lipid-soluble compounds actually contribute significantly to the antioxidant activities and stability of vegetable oils. Tang et al. (2015) showed that the antioxidant activities of lipophilic extracts from the seeds of three colored quinoa cultivars were positively correlated with polyunsaturated fatty acids, total carotenoids and total tocopherols.

Conclusion
D. insignis seed oil is an underutilized plant oil enriched with omega-7 fatty acid. The present study was designed to examine the effect of four different extraction technologies on the yield, physicochemical properties, chemical profile and antioxidant activity of D. insignis seed oil. The results convincingly showed that D. insignis seed oil is distinctive for its unusually large amount of unsaturated fatty acids, especially palmitoleic acid. The oil also contains a high content of phytosterols and polyphenols and exhibits considerable antioxidant activity in vitro. Moreover, ESC-CO2 appears to be more efficient at extracting oil from D. insignis seeds compared with SE, UAE, and SC-CO2 in terms of the quantity and quality characteristics of oils obtained by these methods. Although the present work is the first study to evaluate the extraction efficiency of omega-7-rich oil from D. insignis seeds by the different extract techniques, further experimental investigations are needed to estimate the nutritional value and safety of D. insignis seed oil in vivo and clinically, and another possible area of future research would be to optimize the ESC-CO2 extraction efficiency of the oil in laboratory and industrial scales. Taken together, these results suggest that D. insignis seeds could be considered as a new natural source of palmitoleic acid.
Acknowledgements This research has been funded by the Fundamental Research Funds for the Central Universities (GK201602001), China Postdoctoral Science Foundation (2015M580811), Agriculture Science and Technology Innovation and Research Funds of Shaanxi Province (2016NY-174), and Key Laboratory of Agro-Products Processing, Ministry of Agriculture, P. R. China (2015007).
References
- Belayneh, H. D., Wehling, R. L., Cahoon, E., and Ciftci, O. N. (2015). Extraction of omega-3-rich oil from Camelina sativa seed using supercritical carbon dioxide. J. Supercrit. Fluids, 104, 153-159.
- Bisht, T. S., Sharma, S. K., Sati, R. C., Rao, V. K., Yadav, V. K., Dixit, A. K., Sharma, A. K., and Chopra, C. S. (2015). Improvement of efficiency of oil extraction from wild apricot kernels by using enzymes. J. Food Sci. Tech. Mys., 52, 1543-1551.
- Brahmi, F., Mechri, B., Dabbou, S., Dhibi, M., and Hammami, M. (2012). The efficacy of phenolics compounds with different polarities as antioxidants from olive leaves depending on seasonal variations. Ind. Crops Prod., 38, 146-152.
- Chemat, F., Rombaut, N., Meullemiestre, A., Turk, M., Perino, S., Fabiano-Tixier, A. S., and Abert-Vian, M. (2017). Review of green food processing techniques. Preservation, transformation, and extraction. Innov. Food Sci. Emerg., 41, 357-377.
- Chen, F., Zhang, Q., Gu, H., and Yang, L. (2016). An approach for extraction of kernel oil from Pinus pumila using homogenate-circulating ultrasound in combination with an aqueous enzymatic process and evaluation of its antioxidant activity. J. Chromatogr. A, 1471, 68-79.
- Choudhary, S. P. and Tran, L. S. (2011). Phytosterols: Perspectives in human nutrition and clinical therapy. Curr. Med. Chem., 18, 4557-4567.
- Cunha, S. S., Fernandes, J. O., and Oliveira, M. B. P. P. (2006). Quantification of free and esterified sterols in Portuguese olive oils by solid-phase extraction and gas chromatography-mass spectrometry. J. Chromatogr. A, 1128, 220-227.
- Da Porto, C., Decorti, D., and Natolino, A. (2016). Microwave pretreatment of Moringa oleifera seed: Effect on oil obtained by pilot-scale supercritical carbon dioxide extraction and Soxhlet apparatus. J. Supercrit. Fluids, 107, 38-43.
- da Silva, S. B., dos Santos Garcia, V. A., Arroyo, P. A., and da Silva, C. (2017). Ultrasound-assisted extraction of radish seed oil with methyl acetate for biodiesel production. Can. J. Chem. Eng., 95, 2142-2147.
- de Souza, C. O., Vannice, G. K., Rosa Neto, J. C., and Calder, P. C. (2018). Is palmitoleic acid a plausible nonpharmacological strategy to prevent or control chronic metabolic and inflammatory disorders? Mol. Nutr. Food Res., 62, 1700504.
- Dubois, V., Breton, S., Linder, M., Fanni, J., and Parmentier, M. (2007). Fatty acid profiles of 80 vegetable oils with regard to their nutritional potential. Eur. J. Lipid Sci. Tech., 109, 710-732.
- Ezeh, O., Gordon, M. H., and Niranjan, K. (2016). Enhancing the recovery of tiger nut (Cyperus esculentus) oil by mechanical pressing: Moisture content, particle size, high pressure and enzymatic pre-treatment effects. Food Chem., 194, 354-361.
- Fiorini, D., Boarelli, M. C., Conti, P., Alfei, B., Caprioli, G., Ricciutelli, M., Sagratini, G., Fedeli, D., Gabbianelli, R., and Pacetti, D. (2018). Chemical and sensory differences between high price and low price extra virgin olive oils. Food Res. Int., 105, 65-75.
- Frigolet, M. E. and Gutierrez-Aguilar, R. (2017). The role of the novel lipokine palmitoleic acid in health and disease. Adv. Nutr., 8, 173S-181S.
- Gao, S., Guo, Q., Qin, C., Shang, R., and Zhang, Z. (2017). Sea buckthorn fruit oil extract alleviates insulin resistance through the PI3K/Akt signaling pathway in Type 2 diabetes mellitus cells and rats. J. Agr. Food Chem., 65, 1328-1336.
- Hammann, S. and Vetter, W. (2016). Method development for the determination of free and esterified sterols in button mushrooms (Agaricus bisporus). J. Agr. Food Chem., 64, 3437-3444.
- Hussain, N., Jabeen, Z., Li, Y., Chen, M., Li, Z., Guo, W., and Jiang, L. (2013). Detection of tocopherol in oilseed rape (Brassica napus L.) using gas chromatography with flame ionization detector. J. Integr. Agr., 12, 803-814.
- Hrabovski, N., Sinadinovic-Fiser, S., Nikolovski, B., Sovilj, M., and Borota, O. (2012). Phytosterols in pumpkin seed oil extracted by organic solvents and supercritical CO2. Eur. J. Lipid Sci. Tech., 114, 1204-1211.
- Jin, K., Lee, J., Kwon, H., and Kim, B. (2014). Anti-oxidative and anti-inflammatory activities of Decaisnea insignis ethanol extract. J. Life Sci., 24, 973-980.
- Konopka, I., Roszkowska, B., Czaplicki, S., and Tanska, M. (2016). Optimization of pumpkin oil recovery by using aqueous enzymatic extraction and comparison of the quality of the obtained oil with the quality of cold-pressed oil. Food Technol. Biotech., 54, 413-420.
- Li, B., Lin, F., Huang, P., Guo, W., and Zheng, Y. (2017). Complete chloroplast genome sequence of Decaisnea insignis: Genome organization, genomic resources and comparative analysis. Sci. Rep-UK, 7.
- Li, W., Li, Z., Han, X., Huang, D., Lu, Y., and Yang, X. (2016). Enhancing the hepatic protective effect of genistein by oral administration with stachyose in mice with chronic high fructose diet consumption. Food Funct., 7, 2420-2430.
- Liu, J. and Liu, X. (2011). Fatty acid composition and physiochemical properties of Decaisnea fargesii Franch seed oil. China Oils Fats, 36, 78-79.
- Liu, J., Gasmalla, M. A. A., Li, P., and Yang, R. (2016). Enzyme-assisted extraction processing from oilseeds: Principle, processing and application. Innov. Food Sci. Emerg., 35, 184-193.
- Liu, Z., Gui, M., Xu, T., Zhang, L., Kong, L., Qin, L., and Zou, Z. (2019). Efficient aqueous enzymatic-ultrasonication extraction of oil from Sapindus mukorossi seed kernels. Ind. Crops Prod., 134, 124-133.
- Millao, S. and Uquiche, E. (2016). Extraction of oil and carotenoids from pelletized microalgae using supercritical carbon dioxide. J. Supercrit. Fluids, 116, 223-231.
- Nehdi, I. A., Sbihi, H. M., Tan, C. P., Rashid, U., and Al-Resayes, S. I. (2018). Chemical composition of date palm (Phoenix dactylifera L.) seed oil from six Saudi Arabian cultivars. J. Food Sci., 83, 624630.
- Passos, C. P., Yilmaz, S., Silva, C. M., and Coimbra, M. A. (2009). Enhancement of grape seed oil extraction using a cell wall degrading enzyme cocktail. Food Chem., 115, 48-53.
- Qin, S., Xue, S., Steinberger, Y., Li, G. Y., and Xie, G. H. (2015). Evaluation and screening of potential non-food biodiesel plants from native wild species of northwestern China. J. Biobased. Mater. Bio., 9, 528-536.
- Sahena, F., Zaidul, I. S. M., Jinap, S., Karim, A. A., Abbas, K. A., Norulaini, N. A. N., and Omar, A. K. M. (2009). Application of supercritical CO2 in lipid extraction - A review. J. Food Eng., 95, 240-253.
- Schwartz, H., Ilainen, V., Pfironen, V., and Lampi, M. A. (2008). Tocopherol, tocotrienol and plant sterol contents of vegetable oils and industrial fats. J. Food Compos. Anal., 21, 152-161.
- Slatnar, A., Mikulic-Petkovsek, M., Stampar, F., Veberic, R., and Solar, A. (2015). Identification and quantification of phenolic compounds in kernels, oil and bagasse pellets of common walnut (Juglans regia L.). Food Res. Int., 67, 255-263.
- Sun, X., Gao, G., Yan, B., Zhao, J., Gu, L., and Liu, J. (2012a). Analysis of fatty acids and amino acids in seeds of Akebia trifoliata (Thunb.) Koidz and Decaisnea fargesii Franch. J. Chin. Med. Mater., 35, 1444-1447.
- Sun, X., Duan, A., Gao, G., Yan, B., Liu, J., and Ma, W. (2012b). Optimization of Decaisnea insignis seed oil extraction process and analysis of fatty acid. Sci. Tech. Food Ind., 33, 236-241.
- Suzuki, H., Inoue, T., Fujioka, S., Saito, T., Takatsuto, S., Yokota, T., Murofushi, N., Yanagisawa, T., and Sakurai, A. (1995). Conversion of 24-methylcholesterol to 6-oxo-24-methylcholestanol, a putative intermediate of the biosynthesis of brassinosteroids, in cultured-cells of catharanthus-roseus. Phytochemistry, 40, 1391-1397.
- Tang, Y., Li, X., Chen, P. X., Zhang, B., Hernandez, M., Zhang, H., Marcone, M. F., Liu, R., and Tsao, R. (2015). Characterisation of fatty acid, carotenoid, tocopherol/tocotrienol compositions and antioxidant activities in seeds of three Chenopodium quinoa Willd. genotypes. Food Chem., 174, 502-508.
- Uddin, M. S., Sarker, M. Z. I., Ferdosh, S., Akanda, M. J. H., Easmin, S., Bt Shamsudin, S. H., and Yunus, K. B. (2015). Phytosterols and their extraction from various plant matrices using supercritical carbon dioxide: A review. J. Sci. Food Agr., 95, 1385-1394.
- Vaisali, C., Charanyaa, S., Belur, P. D., and Regupathi, I. (2015). Refining of edible oils: a critical appraisal of current and potential technologies. Int. J. Food Sci. Tech., 50, 13-23.
- Wang, Q., Cheng, J. X., Yan, S. S., Zhao, L. H., and Shao, H. J. (2018a). Recent advances in chemical and potential uses of Decaisnea insignis. Shaanxi J. Agri. Sci., 64, 88-92.
- Wang, Q., Cheng, J. X., Wang, L. X., Yan, S. S., Wang, R. Q., Zhang, H. S., Shao, H. J., and Yang, X. B. (2018b). Valorization of spent shiitake substrate for recovery of antitumor fungal sterols by ultrasound-assisted extraction. J. Food Biochem., 2018, e12602, doi: 10.1111/jfbc.12602.
- Yang, F. Q., Feng, K., Zhao, J., and Li, S. P. (2009). Analysis of sterols and fatty acids in natural and cultured Cordyceps by one-step derivatization followed with gas chromatography-mass spectrometry. J. Pharmaceut. Biomed., 49, 1172-1178.
- Yang, Z., Miyahara, H., and Hatanaka, A. (2011). Chronic administration of palmitoleic acid reduces insulin resistance and hepatic lipid accumulation in KK-A(y) Mice with genetic type 2 diabetes. Lipids Health Dis., 10, 120.
- You, J., Peng, C., Liu, X., J, X., Lu, J., Tong, Q., Wei, P., Cong, L. L., Li, Z. Y., and Huang, H. (2011). Enzymatic hydrolysis and extraction of arachidonic acid rich lipids from Mortierella alpina. Bioresource Technol., 102, 6088-6094.
- Zhou, Z., Shao, H., Han, X., Wang, K., Gong, C., and Yang, X. (2017). The extraction efficiency enhancement of polyphenols from Ulmus pumila L. barks by trienzyme-assisted extraction. Ind. Crops Prod., 97, 401-408.




