2019 Volume 25 Issue 6 Pages 801-807
2019 Volume 25 Issue 6 Pages 801-807
The purpose of this study was to optimize the processing and cooking conditions to retain lutein and reduce the concentrations of oxalate and nitrate in spinach. We investigated the effects of preliminary processing, particularly cutting size and the inclusion of different additives, on changes of oxalate, nitrate, and lutein concentrations in cooked spinach. The results of this study indicated that a combination of boiling in water (100 °C for 2 min) and a 1-cm cutting size resulted in maximal removal of oxalic acid and nitrate ion (72% and 58%, respectively) and maximal retention of lutein (79%) in spinach. Although the inclusion of some adjuvants had significant effects on the concentrations of oxalic acid, nitrate ions and/or lutein at certain concentrations, these effects were not concentration-dependent. We conclude that the addition of various compounds during boiling may not consistently promote either the removal of oxalate and nitrate or lutein retention.
Diets rich in fruits and vegetables are widely recommended in many countries for their health-promoting properties because they supply a range of vitamins, minerals, phytochemicals, and dietary fibers (Slavin et al., 2012). However, some ingredients in fruits and vegetables may have negative effects on human health. Spinach (Spinacia oleracea L.), an important vegetable worldwide, is one of the richest plant sources of lutein, which can protect against age-related macular degeneration and cataracts (Semba et al., 2003; Bartlett et al., 2007; Eisenhauer et al., 2017). In contrast, spinach has been found to accumulate high levels of nitrate and a relatively high concentration of oxalate, which can affect the taste of the product and damage the health of consumers (Holmes et al., 2000; Betsche et al., 2005; Arias-Carmonal et al., 2014).
In most cases, vegetables are cooked prior to consumption because of taste preferences, without regard for the retention of nutrients and health-promoting compounds. Different cooking methods can change the physical characteristics, chemical composition, and concentration and bioavailability of bioactive compounds in vegetables. Boiling in water is the most common cooking method for vegetables. Although harmful ingredients can be removed from vegetables during boiling, health-promoting compounds may also be lost during this process.
In previous studies, many researchers have evaluated the retention of functional compounds processed in boiling water. For instance, Masrizal et al. (1997) quantified changes in concentrations of vitamin C and β-carotene in different vegetables after boiling, microwaving, steaming, and stir-frying. The results demonstrated that vitamin C retention was within the range 34.1%–79.4%, whereas 20%–40% of β-carotene was lost from various vegetables using different cooking methods. A study on broccoli indicated that all cooking methods involving stir-frying or boiling caused significant losses of chlorophyll, vitamin C, and total soluble proteins and sugars (Yuan et al., 2009). Pellegrini et al. (2010) evaluated changes in not only phytochemical content (e.g., carotenoids, chlorophylls, polyphenols, and vitamin C) but also color changes of three widely consumed Brassica oleracea vegetables using different cooking methods. Vitamin C, in particular, was lost in large amounts in all the vegetables. Kojima et al. (2017a, b) reviewed the loss of fat-soluble vitamins (vitamins A, D, E, and K), and the water-soluble vitamins, namely the vitamin B complex (niacin, pantothenic acid, biotin, and folic acid), and vitamin C from various food categories (e.g., meats, fruits, and vegetables) under different cooking conditions. Interestingly, the losses of fat-soluble vitamins during cooking were limited, whereas boiling had a significant effect on water-soluble vitamins, such as niacin and folic acid.
Cooking and food preparation steps can also help to eliminate undesirable vegetable components. According to the study of Izumi et al. (2005), greater amounts of boiling water used in cooking led to greater decreases in the concentration of oxalic acid in spinach. Bong et al. (2017) investigated the effect of adding different types of calcium salts (calcium chloride dehydrates, calcium carbonate, calcium citrate tetrahydrate, and calcium sulfate dehydrate) on the concentration of soluble oxalate in fresh spinach juice. They reported that calcium chloride was the most effective additive in reducing soluble oxalate contents in fresh spinach juice. Our previous study had focused on the effects on oxalate, nitrate, and lutein concentrations of cooking at different temperatures, where we proposed a cooking regime (100 °C for 2 min) under which oxalate, and nitrate ions could be removed from spinach while simultaneously maximizing the extent of lutein retention (Wang et al., 2018). Recently, cutting vegetables into small pieces and eating the raw, so-called chopped salad, has become popular in the United States owing to its ease of eating. In addition, in Japan, a side dish of boiled spinach cut into 4- to 5-cm pieces and seasoned with soy sauce, is popular in the daily diet. Thus, the cutting size may become an important factor affecting the chemical composition of vegetables.
The objective of the present study was to determine the effects of preliminary processing (cutting into smaller pieces) and the addition of small amounts of different compounds (e.g., calcium and magnesium salts) on oxalate, nitrate, and lutein concentrations in spinach. Our goal was to identify the preparation and cooking conditions (e.g., cutting size and use of different additives) that would simultaneously maximize the retention of lutein and the reduction of oxalate and nitrate concentrations in spinach.
Materials Fresh spinach (Spinacia oleracea unknown cultivar (Experiment 1) and “Sanhopu” (Experiment 2)), grain vinegar (Mitsukan, Aichi, Japan), and sugar (Mitsui seito, Tokyo, Japan) were purchased from a local supermarket. Standards of lutein, oxalic acid, and potassium nitrate, as well as sodium hydroxide, 2,6-pyridinedicarboxylic acid, calcium chloride, magnesium chloride, magnesium sulfate, sodium bicarbonate, and ethanol were purchased from Wako Pure Chemical Industries (Osaka, Japan). Hexadecyltrimethylammonium bromide (CTAB) (purity ≥99.0%) was purchased from Nacalai Tesque Inc. (Kyoto, Japan). Pyrogallol and organic solvents were of certified HPLC or analytical grade and purchased from Kanto Chemical Co. Inc. (Tokyo, Japan).
Sample preparation Fresh spinach, either whole (20–30 g/spinach) and/or cut into pieces (1-, 2-, or 4-cm) were used in this study, with five replicates of each treatment. Spinach was boiled in either 3 L of boiling water with no additives or 3 L of boiling water containing different amounts of individual additives (calcium chloride, magnesium chloride, magnesium sulfate, sodium bicarbonate, sodium chloride, vinegar, sugar, and ethanol) in a commercial pot for 2 min. For uncut spinaches, five were boiled for each treatment, whereas for spinach pieces, one spinach was cut up and boiled to represent one replicate. Because the temperature decreased when the spinach was added to the boiling water, the 2-min cooking time was indicated to begin when the water began to boil after addition of the spinach. The boiled spinach was rinsed under running cold water (<1 min), and excess water was removed by a hand squeezing. All samples were stored at −20 °C until analysis. Details of the extraction and determination of oxalate, nitrate, and lutein concentration were described in our previous paper (Wang et al., 2018).
Statistical analysis Statistical analysis was carried out using EXCEL Statistics version 7.0 (Esumi Co., Ltd., Tokyo, Japan). One-way analysis of variance (ANOVA), with the Tukey–Kramer multiple comparison test, was used for comparisons between the control group (boiling water) and treatments with different additives (calcium chloride, magnesium chloride, magnesium sulfate, sodium bicarbonate, sodium chloride, vinegar, sugar, and ethanol) in Experiment 1. Two-way repeated-measures ANOVA was used to analyze the effects of different concentrations of sodium chloride and the cutting process on oxalate, nitrate, and lutein contents at a significance level of P < 0.05 in Experiment 2.
Experiment 1. Effects of additives Our objective was to optimize the preparation and cooking conditions in order to retain lutein and reduce oxalate and nitrate concentrations in spinach. A previous study had reported that the addition of calcium compounds could reduce concentrations of soluble oxalate in vegetable juice (Bong et al., 2017). In the current study, eight different compounds, including calcium salts, magnesium salts, and commercial seasonings, were selected to determine whether the addition of any of these compounds during cooking could change the concentrations of oxalate and nitrate in whole spinach following cooking. We selected these compounds because they are commonly used as either seasonings or food additives (Evaluations of the Joint FAO/WHO Expert Committee on Food Additives (JECFA)). Our previous research had demonstrated that boiling spinach in water at 100 °C for 2 min was the optimal cooking condition to maximally retain lutein and to minimize oxalate and nitrate concentrations (Wang et al., 2018), so we used this optimal cooking regime in the current study. For removal of oxalate, we mainly focused on water-soluble oxalic acid, not insoluble oxalic acid, because the former has more negative effects on health and taste than does the latter (Horie et al., 2006).
The uncut fresh spinach (Spinacia oleracea, unknown cultivar) used in this experiment was cultivated in Ibaraki Prefecture and purchased from a local supermarket on October 2, 2017. The mean ± SE concentration of soluble oxalic acid in the raw spinach was 503.7 ± 20 mg/100 g fresh weight (FW). The concentration of oxalic acid in spinach is affected by many factors, including variety, cultivation conditions, and harvest time. Izumi (2004) reported that the concentration of oxalic acid in spinach, for the same variety, producer, and soil, varied significantly (500–800 mg/100 g FW) under different cultivation seasons and areas. Izumi et al. (2008) reported that the oxalate concentration in spinach decreased during the growing period under all cultivation conditions. Table 1 shows the changes in soluble oxalic acid concentration in whole spinach after boiling in water with different amounts of calcium salts, magnesium salts, or commercial seasonings. Using cooking in unadulterated boiling water as a control, a significant decrease in oxalic acid concentration was observed by adding certain chemical compounds at particular concentrations (e.g., 2 mg/mL MgCl2). However, the effect of such compounds on the change in oxalic acid concentration was not concentration-dependent. Therefore, we considered that the addition of such compounds had no consistent effect on oxalic acid removal. More than 50% of the oxalic acid was removed after boiling at 100 °C for 2 min. This result was consistent with the findings from our earlier study (Wang et al., 2018) and from previous research by Izumi et al. (2005). We also found that the addition of vinegar resulted in browning of the spinach. This can be explained by the finding of Quach et al. (2004) that chlorophyll is transformed into pheophytin after treatment with a weak acid due to the removal of the central Mg2+ ion and its replacement by H+, suggesting that a weak acid (such as acetic acid (vinegar) in the present study) is not suitable for removal of oxalic acid from spinach.
| Processing conditions | mg/100 g FW | |||
|---|---|---|---|---|
| Oxalic acid | Nitrate ion | Lutein | ||
| Raw | 503.7 ± 20.0 | 572.8 ± 19.4 | 13.5 ± 0.23 | |
| Boiling water (Control) | 216.0 ± 14.4 | 327.8 ± 16.4 | 10.7 ± 0.25 | |
| CaCl2 (mg/mL) |
0.5 | 260.1 ± 9.6 | 379.8 ± 11.0 | 10.5 ± 0.33 |
| 1 | 252.3 ± 13.8 | 378.7 ± 22.2 | 10.2 ± 0.32 | |
| 2 | 259.5 ± 13.7 | 381.5 ± 14.8 | 10.4 ± 0.15 | |
| 3 | 257.5 ± 29.6 | 375.4 ± 23.1 | 10.7 ± 0.27 | |
| 4 | 285.0 ± 27.1 | 373.3 ± 26.0 | 11.0 ± 0.50 | |
| 5 | 251.1 ± 16.2 | 370.2 ± 22.6 | 10.2 ± 0.31 | |
| MgCl2 (mg/mL) |
0.5 | 250.4 ± 8.8 | 247.7 ± 19.0* | 11.1 ± 0.29 |
| 1 | 247.3 ± 7.5 | 194.2 ± 8.1* | 1.0 ± 0.23 | |
| 2 | 214.1 ± 17.9 | 255.6 ± 19.3* | 10.3 ±0.36 | |
| 3 | 208.7 ± 11.9 | 340.5 ± 14.7 | 10.5 ± 0.50 | |
| 4 | 226.4 ± 8.8 | 327.7 ± 16.6 | 11.3 ±0.11 | |
| 5 | 189.4 ± 9.7 | 272.5 ± 17.0 | 10.9 ± 0.08 | |
| MgS04 (mg/mL) |
0.5 | 227.7 ± 23.4 | 288.5 ± 11.5 | 10.3 ± 0.21 |
| 1 | 177.5 ± 7.9 | 333.4 ± 20 | 10.8 ± 0.27 | |
| 2 | 188.0 ± 9.5 | 322.9 ± 9.8 | 11.0 ± 0.35 | |
| 3 | 229.2 ± 7.6 | 326.9 ± 17.4 | 10.7 ± 0.24 | |
| 4 | 251.2 ± 15.6 | 274.3 ± 16.4 | 10.5 ± 0.21 | |
| 5 | 236.8 ± 12.5 | 341.5 ± 21.0 | 11.0 ± 0.36 | |
| NaHCO3 (mg/mL) |
0.5 | 247.3 ± 18.5 | 295.6 ± 18.5 | 10.4 ± 0.41 |
| 1 | 254.9 ± 29.8 | 303.5 ± 16.3 | 11.4 ± 0.42 | |
| 2 | 217.2 ± 17.2 | 289.4 ± 12.8 | 10.7 ± 0.31 | |
| 3 | 177.4 ± 14.5 | 256.4 ± 146.6* | 9.7 ± 0.16 | |
| 4 | 208.1 ± 17.8 | 371.7 ± 16.3 | 10.5 ± 0.20 | |
| 5 | 203.6 ± 15.9 | 294.5 ± 19.5 | 10.1 ± 0.27 | |
| NaCl (mg/mL) |
10 | 292.0 ± 25.8 | 340.7 ± 13.5 | 10.9 ± 0.39 |
| 20 | 381.3 ± 36.2* | 355.1 ± 20.2 | 10.0 ± 0.15 | |
| 30 | 458.4 ± 14.2* | 482.9 ± 9.8* | 9.3 ± 0.08 | |
| Sugar (mg/mL) |
10 | 249.0 ± 15.9 | 379.4 ± 12.5 | 11.1 ± 0.29 |
| 20 | 269.9 ± 34.4 | 338.4 ± 19.8 | 10.4 ± 0.14 | |
| 30 | 275.1 ± 26.2 | 359.2 ± 9.9 | 10.8 ± 0.22 | |
| 40 | 260.9 ± 16.1 | 309.5 ± 29.3 | 10.6 ± 0.20 | |
| 50 | 284.4 ± 25.6 | 353.8 ± 27.7 | 11.1 ± 0.41 | |
| Vinegar (%, v/v) |
1 | 275.2 ± 41.2 | 367.5 ± 31.0 | 11.8 ± 0.30 |
| 2 | 262.5 ± 11.9 | 346.6 ± 25.6 | 11.3 ±0.24 | |
| 3 | 268.7 ± 12.8 | 341.8 ±10.3 | 12.2 ± 0.27* | |
| 4 | 248.3 ± 17.8 | 329.3 ± 17.0 | 11.9 ± 0.21* | |
| 5 | 233.2 ± 10.7 | 317.0 ± 14.1 | 11.1 ± 0.23 | |
| Ethanol (%, v/v) |
1 | 203.1 ± 8.6 | 322.1 ± 8.5 | 10.3 ± 0.15 |
| 2 | 276.5 ± 5.4* | 272.7 ± 27.3 | 11.5 ± 0.62 | |
| 3 | 262.2 ± 17.2 | 244.3 ± 10.3* | 10.6 ± 0.20 | |
| 4 | 270.2 ± 10.5* | 288.3 ± 6.9 | 11.2 ± 0.20 | |
| 5 | 216.7 ± 11.3 | 329.9 ± 9.7 | 11.1 ± 0.12 | |
The mean ± SE concentration of nitrate ions in raw spinach was 572.8 ± 19.4 mg of 100 g FW. Nitrate ion concentration in spinach can be affected by variety, environmental conditions, nitrogen fertilization regime, temperature, season and other factors (Okazaki et al., 2006; Ryu et al., 2007). The changes in nitrate ion concentration in spinach under different boiling conditions are shown in Table 1. Approximately 40% of the nitrate ions were removed under all conditions. However, we found that the addition of different compounds did not result in significantly greater removal of nitrate ions during boiling when compared with boiling water alone. There are two perspectives on the effect of dietary nitrate ions on human health. Some researchers have determined that high concentrations of nitrate ions can cause infantile methemoglobinemia, and increase the frequency of cancer, and other diseases (Pennington, 1998; Sanchez-Echaniz et al., 2001). In contrast, nitrate ions have also been shown to have health benefits, such as anti-inflammatory effects (Delmastro-Greenwood et al., 2015). We will not discuss these perspectives further because concentrations of both oxalic acid and nitrate ion were significantly reduced during boiling.
Oowashi et al. (2014) reported that the concentration of lutein in spinach could be affected by cultivar and environmental conditions. In the current study, the mean ± SE concentration of lutein in raw spinach was 13.5 ± 0.23 mg/100 g FW. Approximately 80% of the lutein content was retained after boiling under all conditions (Table 1). Interestingly, our results indicate that the addition of sodium chloride at various concentrations did not improve the retention of lutein in spinach although the color of spinach boiled with sodium chloride was bright green. This may not have been due to the addition of salt because a similar study by Lanman et al.(1927) reported that the color of vegetables was not noticeably changed when pure sodium chloride was added at various times during the cooking process.
Experiment 2. Effect of cutting process and/or addition of culinary salt Based on the findings from Experiment 1, we found no beneficial effect of adding any of the compounds on either lutein retention or the removal of oxalic acid and nitrate ions from whole spinach during boiling. Subsequently, we investigated the effect of cutting spinach into pieces on the concentration of lutein, oxalic acid, or nitrate ions under the same boiling condition as above (100 °C for 2 min). Specifically, we cut the spinach into 1-, 2-, or 4- cm pieces. We expected that the presence of cut edges would increase the removal of the water-soluble oxalic acid and nitrate ions in boiling water. We also added 1% and 2% sodium chloride to the boiling water to determine whether the effect of salt on the decrease in oxalate and nitrate concentrations in whole spinach was also evident in cut spinach.
A late-bolting cultivar of fresh spinach (Spinacia oleracea “Sanhopu”), cultivated in Gifu Prefecture and purchased from a local supermarket on June 4, 2018, was used in Experiment 2. The mean ± SE concentration of oxalic acid in this raw spinach was 412.7 ± 6.7 mg/100 g FW, which differed from that in Experiment 1 (503.7 ± 20 mg/100 g FW), which may be due to the different cultivation site (Ibaraki Prefecture vs. Gifu Prefecture) or season of cultivation (October vs. June). In addition, based on appearance, the two batches of spinach appeared not to be the same cultivar (cultivar of spinach used in Experiment 1 was unknown), which may also explain the different concentrations of oxalic acid in the raw plants. After boiling spinach at 100 °C for 2 min, the oxalic acid concentration decreased significantly, regardless of the addition of sodium chloride (Fig. 1). Two-way repeated-measures ANOVA assessed the effects of different cutting process and the addition of sodium chloride on oxalic acid removal, although there was no significant interaction between cutting size and sodium chloride (P > 0.05). All pairwise comparisons using the Tukey–Kramer test further indicated significant differences among each treatment (P < 0.05). The reduction in oxalic acid was closely related to cutting size, with the narrower pieces resulting in greater removal of oxalic acid. As shown in Figure 1, the mean ± SE oxalic acid concentration in cooked whole spinach was 189.1 ± 18.6 mg/100 g FW, which decreased in spinach cut to 4- or 2-cm pieces, reaching its lowest level of 117.1 ± 2.9 mg/100 g FW in spinach cut to a 1-cm pieces. We demonstrated that the addition of sodium chloride did not further increase the removal of oxalic acid when compared with boiling water alone, regardless of cutting size, a finding consistent with the results of Experiment 1 (Fig. 1).
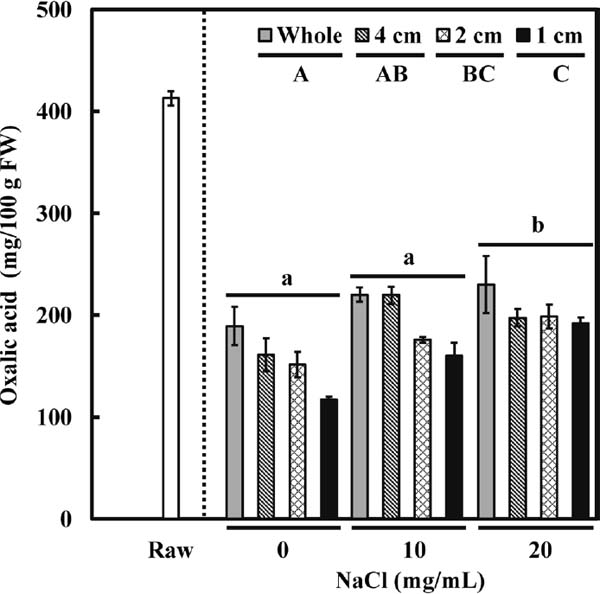
Effect of processing and cooking conditions (cutting into different sizes and the addition of sodium chloride at different concentrations) on changes in oxalic acid concentration (mg/100 g FW). All values are mean ± SE (n = 5); any two samples with a common letter are not significantly different (P > 0.05). Uppercase letters represent cutting size and lowercase letters the addition of sodium chloride (Tukey–Kramer test).
Figure 2 shows the changes in nitrate ion content. As compared with raw spinach, which had a nitrate ion concentration of 421.6 ± 11.3 mg/100 g FW, boiled whole spinach had a concentration of 264.9 ± 2.9 mg/100 g FW (Fig. 2). Similar to the results concerning oxalic acid concentration, there was no interaction between cutting size and sodium chloride addition on the nitrate ion concentration. As shown in Fig. 2, a smaller leaf piece resulted in greater loss of nitrate ions. However, unlike the situation with oxalic acid, the concentration of nitrate ions was not affected by the addition of sodium chloride. Figure 3 shows that the concentration of lutein in whole spinach decreased from 19.2 ± 0.1 to 15.3 ± 0.3 mg/100 g FW after boiling. Compared with whole spinach, the concentration of lutein did not significantly decrease with a cutting size of 4- or 2-cm, but with a cutting size of 1 cm (15.2 ± 0.5 mg/100 g of FW). However, the 1-cm-cutting tended to affect the lutein concentration only after the addition of sodium chloride, although the two main factors (cutting size and addition of sodium chloride) did not show a significant interactive effect on the concentration of lutein in two-way ANOVA. Interestingly, unlike the results for the water-soluble components oxalic acid and nitrate ions, the concentration of lutein decreased further with addition of salt during boiling, particularly at a cutting size of 1 cm. Our results indicate that boiling spinach in the presence of sodium chloride can accelerate the leaching of lutein.
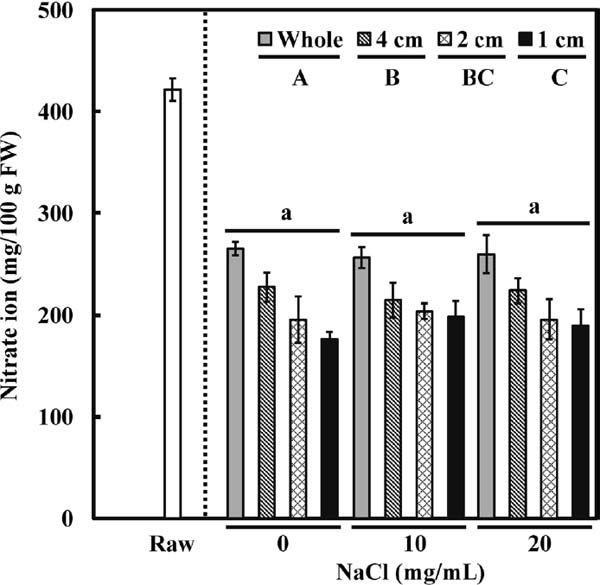
Effect of processing and cooking conditions (cutting into different sizes and the addition of different sodium chloride concentrations) on changes in nitrate ion concentration (mg/100 g FW). All values are mean ± SE (n = 5); any two samples with a common letter are not significantly different (P > 0.05). Uppercase letters represent cutting size and lowercase letters represent sodium chloride addition (Tukey–Kramer test).
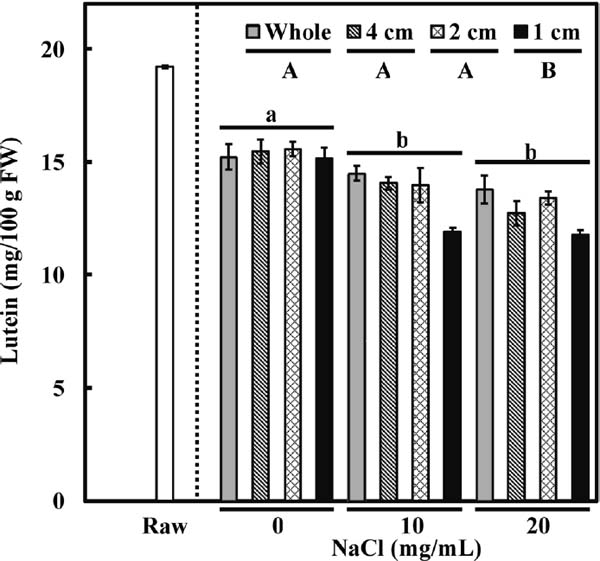
Effect of processing and cooking conditions (cutting into different sizes and addition of different concentrations of sodium chloride) on changes in lutein concentration (mg/100 g FW). All values are mean ± SE (n = 5); any two samples with a common letter are not significantly different (P > 0.05). Uppercase letters represent cutting size and lowercase letters represent sodium chloride addition (Tukey–Kramer test).
The spinach used in these two experiments had different origins and harvest times, which may have affected the concentrations of oxalic acid, nitrate ion, and lutein in them. However, our results consistently indicated that the percentage removal (oxalic acid and nitrate ion) and percentage retention (lutein) were similar in both experiments after treatment under the same boiling conditions: 57% of oxalic acid and 43% of nitrate ion were removed in spinach (unknown cultivar) cultivated in Ibaraki Prefecture and purchased on October 2, 2017, whereas 54% of oxalic acid and 37% of nitrate ion were removed in spinach “Sanhopu” cultivated in Gifu Prefecture and purchased on June 4, 2018. These results were in close agreement with our previous research (Wang et al., 2018) with spinach “Jasuteisu” cultivated in the Ibaraki Prefecture and purchased on June 27, 2017, in which 67% of oxalic acid and 30% of nitrate ions were removed after 2 min at 100 °C.
In conclusion, our results suggest that a combination of boiling in water (100 °C for 2 min) and cutting spinach to 1-cm can maximize the removal of oxalic acid and nitrate ion (by 72% and 58%, respectively; P < 0.05) and the retention of lutein (79%; P < 0.05) in spinach. The addition of compounds (e.g., sodium chloride) during boiling did not promote the removal of oxalic acid or nitrate ions, whereas it could increase loss of lutein regardless of cutting size.
The percentage removal (oxalic acid and nitrate ion) and retention (lutein) after boiling whole spinach were fairly similar between the current study and our previous report (Wang et al., 2018), ranging from 54% to 67% for oxalic acid removal, 30% to 43% for nitrate ion removal, and 77% to 80% for lutein retention, even if the background of the spinach (e.g., cultivar, cultivation, and harvest time) was completely different, suggesting a robustness of the effects, regardless of the background of spinach. Our results may be used as a reference for similar studies in the future.
Acknowledgements This research was supported by grants from the Project of the NARO Bio-oriented Technology Research Advancement Institution (the special scheme project on regional developing strategy). We also thank Dr. Takayuki Mitsunaga of the national agricultural research and center, NARO, for the professional advice regarding statistical analysis.