2019 Volume 25 Issue 6 Pages 809-815
2019 Volume 25 Issue 6 Pages 809-815
We previously reported that Citrus kawachiensis peel is abundant in auraptene (AUR), 3,5,6,7,8,3′,4′-heptamethoxyflavone (HMF), nobiletin (NBT), naringin (NGIN), and narirutin (NRTN). All of the above are known as citrus functional components, and a part of these is extracted from the peel into the juice during fruit squeezing. In the present study, we demonstrated that while squeezing method did not affect the composition of compounds in the juice, the contents were affected. Heat treatment for a longer duration led to AUR decomposition, while HMF, NBT, NGIN, and NRTN did not decompose. These 5 components were stable in hot ethanol after 24 h.
Citrus kawachiensis Hort. ex. Y. Tanaka (Japanese name, Kawachi bankan), a late-ripening citrus species in Japan, is a cultivar of Citrus paradisi (grapefruit) (Sawamura, 2000). According to the annual report of the Ministry of Agriculture, Forestry and Fisheries, Japan, a total of 6,823 tons of C. kawachiensis was produced in 2016, mostly in Ehime Prefecture.i
We previously demonstrated that 1) the peel of C. kawachiensis is a rich source of auraptene (AUR), 3,5,6,7,8,3′,4′-heptamethoxyfla-vone (HMF), and naringin (NGIN); 2) single administration of HMF, AUR or NGIN, exerts neuroprotective effects in the central nervous system (CNS); 3) the fruit's dried peel powder also registers neuroprotective effects on the CNS; 4) a part of these components are extracted from the peel into the juice during fruit squeezing; and 5) both AUR and HMF pass through the blood-brain-barrier (BBB) (Furukawa et al., 2012; Amakura et al., 2013; Okuyama et al., 2013; Okuyama and Morita et al., 2014; Okuyama and Yamamoto et al., 2014; Okuyama and Miyoshi et al., 2015; Okuyama and Morita et al., 2015; Okuyama et al., 2016; Sawamoto et al., 2016; Okuyama et al., 2017; Sawamoto et al., 2017; Okuyama and Kotani et al., 2018; Okuyama and Nakashima et al., 2018; Okuyama and Yamamoto et al., 2018; Okuyama and Shinoka et al., 2018).
Based on these findings, AUR-rich C. kawachiensis fruit juice was prepared by adding peel paste to the raw juice. The study succeeded in clarifying the therapeutic potential of the prepared fruit juice in ischemic mice (by preventing ischemia-induced neuronal cell death through anti-inflammatory responses) (Okuyama et al., 2019) and healthy human volunteers (by checking age-derived cognitive decline) (Igase et al., 2017).
In commercial citrus juice manufacturing, several processing methods exist, such as the JBT (John Bean Technologies) (formerly FMC, commonly called “in-line”), chopper pulper, belt-press, and centrifugation extractions. Previously, several studies have revealed that the extraction method influences the content of bioactive compounds in citrus juice. For example, in orange juice manufactured using various methods (squeezing, mild pasteurization, standard pasteurization concentration, and freezing), phenolic compounds, vitamin C, and antioxidant activity have been reported (Gil-Izquierdo et al., 2002). Similarly, polymethoxyflavones [tangeretin, nobiletin (NBT), and sinensetin] and β-cryptoxanthin concentrations in ponkan (Citrus reticulata) juices, processed by in-line, chopper pulper, and hand-press extractions, have been reported (Nogata et al., 2003). Additionally, the color, particle size, carotenoid content, and its bioaccessibility in the processed and hand-squeezing orange juices were evaluated (Stinco et al., 2012). On the other hand, comparisons of data according to industrial production-line are scarce.
C. kawachiensis juice was prepared using typical squeezing methods for citrus fruits, i.e., belt-press and JBT citrus extractors. A belt-press extractor slowly and completely ruptures the peel oil cells, resulting in an increased peel oil content in the juice (Ohta et al., 1983). In commercial production of functional C. kawachiensis fruit juice, the content and stability of functional ingredients such as AUR and HMF are important. Therefore, this study aimed to investigate the effect(s) of two processing methods on the content of representative components of C. kawachiensis, i.e., AUR, HMF, NGIN, NBT, and narirutin (NRTN). To analyze the stability of the above components, the fruit juice was exposed to heat and alcohol treatment.
Samples and reagents C. kawachiensis fruits, harvested from Yawatahama (Ehime, Japan) in June 2014, were squeezed using a belt-press citrus juice extractor (Model No. SFP-2200; IKAWA IRON WORKS CO., LTD., Tokushima, Japan) or a JBT (formerly FMC) citrus juice extractor (Model No. 291/391; John Bean Technologies Corporation, Chicago, IL, USA) to obtain the juice. From the fruits, 35.4 and 54.0% (v/w) juice was obtained with the belt-press and JBT juice extractors, respectively. Standard substances for identification, such as AUR, HMF, NGIN, NBT, NRTN, coniferin, isoconiferin, narirutin 4′-O-β-D-glucoside, naringin 4′-O-β-D-glucoside, syringin, marmin, 6′,7′-dihydroxybergamottin, and bergamottin, were isolated from C. kawachiensis peel as previously described (Amakura et al., 2013). Standard compounds for the quantitative analysis of AUR, HMF, NGIN, NBT, and NRTN were procured from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). All other materials used were also of reagent grade.
Assessment of juice ingredients The juice (50 µL each, 2 samples) was added to identical volumes of methanol (MeOH), sonicated, and filtered (0.22 µm) to produce the sample solutions. Standard solutions, prepared in MeOH, were diluted to obtain a calibration curve with linearity in the range of 0.1–10 µg/mL. High-performance liquid chromatography (HPLC) analysis was performed with a Shimadzu Prominence system (Shimadzu, Kyoto, Japan). Reversed phase (RP)-HPLC conditions were as follows: column (L-column ODS; 5 µm, 150 × 2.1 mm I.D.) (Chemicals Evaluation and Research Institute, Tokyo, Japan); mobile phase, 5% acetic acid (solvent A) and acetonitrile (solvent B) (0–30 min, 0–50% B in A; 30– 35 min, 50–85% B in A; 35–40 min, 85% B in A; 40–50 min, 85–90% B in A; 50–55 min, 90–100% B in A; 55–60 min, 100% B in A); injection volume, 2 µL; column temperature, 40 °C; flow-rate, 0.3 mL/min; detection at 320 nm (AUR), 340 nm (HMF), 330 nm (NBT), and 280 nm (NGIN and NRTN).
Heating decomposition of juice and citrus constituents The juice (2 mL) was poured into a capped tube, which was later sealed. HPLC was performed after 1, 3, 6, and 10 h in a boiling water bath (for 3 samples). The juice, after heat decomposition, was subjected to column chromatography with a YMC GEL ODS-AQ (AQ12S50, 36 × 1.1 cm I.D.) (YMC, Kyoto, Japan) and aqueous MeOH to obtain 5-hydroxymethyl-2-furaldehyde (5.9 mg) (Kulkarni et al., 2008). 1H- and 13C-NMR spectra were recorded on a Bruker Avance 500 (Bruker BioSpin Co., Billerica, MA, USA) (500 MHz for 1H and 126 MHz for 13C) and chemical shifts were provided in ppm values relative to that of the solvent [MeOH-d4 (δH 3.30; δC 49.0)] on a tetramethylsilane scale. 5-Hydroxymethyl-2-furaldehyde: 1H-NMR (MeOH-d4) δ 9.53 (1H, s, -CHO), 7.37 (1H, d, J=3.5 Hz, H-3), 6.57 (1H, d, J=3.5 Hz, H-4), 4.60 (2H, s, H-6). 13C-NMR (MeOH-d4) δ 153.9 (C-2), 124.8 (C-3), 110.9 (C-4), 163.2 (C-5), 57.6 (C-6), 179.4 (-CHO). Each citrus constituent (AUR, HMF, NBT, NGIN, and NRTN) in ethanol (1.0 mg/mL) (2 mL) was sealed in a capped tube. HPLC analyses were performed after 1, 3, 6, 10, and 24 h in a water bath (∼80 °C).
Analysis of juice ingredients To obtain C. kawachiensis juice, fruits were commercially squeezed by belt-press and JBT citrus juice extractors. In the present study, 35.4 or 54.0% (v/w) juice was obtained from the fruits with the belt-press and JBT juice extractors, respectively. We have previously identified 15 compounds from the ethanolic extract of C. kawachiensis peel (Amakura et al., 2013). To estimate the influence of squeezing methods on the content of juice ingredients, juice extracts were analyzed by RP-HPLC as previously described (Amakura et al., 2013). Figures 1 (A) and (B) display the HPLC chromatograms of the juices squeezed by belt-press and JBT juice extractors, respectively. The results revealed that 13 compounds, i.e., coniferin, isoconiferin, narirutin 4′-O-β-D-glucoside, naringin 4′-O-β-D-glucoside, syringin, NRTN, NGIN, marmin, 6′,7′-dihydroxybergamottin, NBT, HMF, AUR, and bergamottin, which are present in the peel extract, were also present in the juices. Figures 1 (A) and (B) also present NGIN as the main component in both juices, followed by NRTN.

HPLC chromatograms of the juice prepared by (A) belt-press extractor and (B) JBT extractor (detected at 280 nm). Chemical structures of the identified compounds were indicated in (A).
Among the 13 compounds present in the juice extracts, AUR, HMF, NBT, and NGIN have been speculated to play an important role in neuroprotective effects (Onozuka et al., 2008; Furukawa et al., 2012; Okuyama et al., 2013; Nakajima et al., 2013; Okuyama and Morita et al., 2014; Okuyama and Yamamoto et al., 2014; Okuyama and Miyoshi et al., 2015; Okuyama and Morita et al., 2015; Okuyama et al., 2016; Sawamoto et al., 2016; Okuyama et al., 2017; Sawamoto et al., 2017; Okuyama and Nakashima et al., 2018); whereas, NRTN has been reported to possess anti-allergic effects (Funaguchi et al., 2007). The contents of the above-listed 5 compounds in both juice samples were assessed. To prepare an RP-HPLC test sample, the juice was added to the same MeOH volume, sonicated, and filtered. Standard solutions were prepared in MeOH and diluted to obtain a calibration curve with linearity in the range of 0.1–10 µg/mL.
In the belt-press extracted juice, the AUR content was 2-times higher than in the JPT extracted juice (Figure 2). Additionally, HMF and NBT contents were also approximately 7-times higher in the belt-press squeezed juice than in the JBT extracted juice. In contrast, NGIN and NRTN contents in the belt-press extracted juice were lower than in the JBT extracted juice. The reason for this discrepancy could be attributed to the fact that lipophilic compounds (AUR, HMF, and NBT) were effectively extracted with the belt-press juice extractor by applying strong pressure on the peel, which is in good agreement with the characteristic of the two juice extractors.

Auraptene (AUR), 3,5,6,7,8,3′,4′-heptamethoxyflavone (HMF), nobiletin (NBT), naringin (NGIN), and narirutin (NRTN) contents in the juice squeezed by (A) belt-press extractor and (B) JBT extractor. Each data was expressed as the mean of three times analyses.
The findings revealed that 1) 13 peel components were transferred to the juice by both squeezing methods; however, 2) contents of the components in the juice depended on the squeezing method, suggesting that either of the squeezing methods could be selected depending on the intended purpose.
Stability of citrus constituents The thermal stability of 5 components (AUR, HMF, NBT, NGIN, and NRTN) in the juice was analyzed. The belt-press extracted juice was poured into a capped tube and placed in a boiling water bath for 0, 1, 3, 6, and 10 h. Figure 3 presents typical chromatograms of the sample after 0, 3, and 6 h of heat treatment. The patterns observed at 3 and 6 h were almost similar to that at 0 h. Figure 3 reveals a gradual decrease in the AUR peak between 3–6 h, and the detection of a new peak (Peak A) in response. Figure 4 presents the temporal changes in the contents of 5 juice components during the heat treatment, and indicates that heat-treatment of the juice for more than 3 h resulted in AUR decomposition. In contrast, the remaining 4 components, i.e., HMF, NBT, NGIN, and NRTN, were noted as stable after 10 h.

HPLC (detected at 280 nm) chromatogram of the juices following the heat treatment. The chemical structure of 5-hydroxymethyl-2-furaldehyde (identified as the product of heat decomposition) is indicated.

Time course of the content ratio of citrus constituents in the heat-treated juice. Each data was expressed as the mean of six times analyses.
To identify the compound constituting Peak A, the heat-treated juice was subjected to column chromatography with a YMC GEL ODS-AQ and aqueous MeOH for compound isolation. This was followed by NMR identification to reveal the compound as 5-hydroxymethyl-2-furaldehyde, a known major anti-oxidative component of barley tea (Kulkarni et al., 2008) and a constituent of honey, etc. (Castoldi et al., 2016). 5-Hydroxymethyl-2-furaldehyde has also been reported as a product of the heat decomposition of sugar (Urashima et al., 1983); therefore, its presence in the heated juice is likely attributable to sugar decomposition.
Furthermore, the thermal stabilities of AUR, HMF, NBT, NGIN, and NRTN in alcohol were individually analyzed as a simulation of liqueur products, etc. When each citrus constituent in ethanol was sealed in a capped tube and placed in a water bath (∼80 °C) for 1, 3, 6, 10, and 24 h, the content of all 5 components did not change, even after 24 h of treatment (Figure 5).
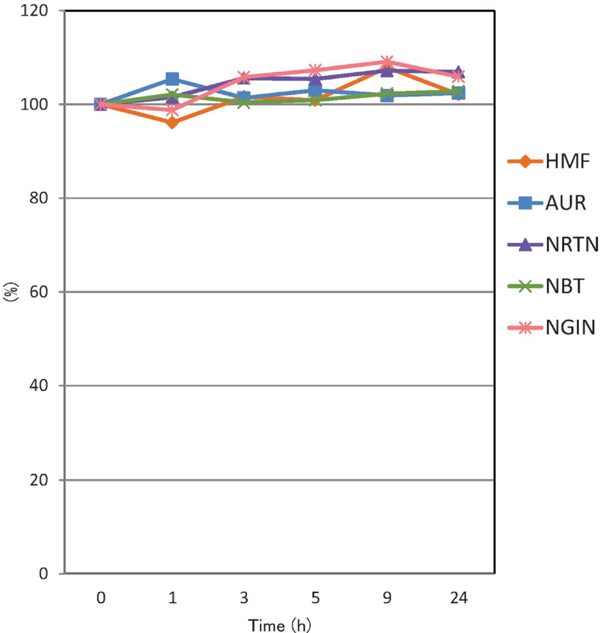
Time course of the content ratio of AUR, HMF, NBT, NGIN, and NRTN in ethanol. Each data was expressed as the mean of six times analyses.
We investigated the components of C. kawachiensis fruit juice prepared by belt-press and JBT juice extractors using RP-HPLC analysis. As a result, 13 peel compounds were identified in both juice types; however, the contents were dependent on the squeezing method. When considering only the production efficiency of the juice, the JBT juice extractor was observed to be effective because of the high yield (54.0%). On the other hand, if functional components are evaluated, a method could be selected based on the results obtained in this research. The heat stability of the juice components (AUR, HMF, NBT, NGIN, and NRTN) was investigated in a boiling water bath; AUR decomposition was noted as about 10% after 3 h, whereas, all other components were stable after 10 h. During heat treatment, 5-hydroxymethyl-2-furaldehyde (speculated to be a product of sugar decomposition) was observed. The thermal stability of the 5 components in ethanol was also investigated; none decomposed in ethanol after 24 h. Together, these findings suggested that 1) the squeezing method should be applied appropriately depending on the purpose; 2) the heat treatment, if necessary, should not exceed 1 h.
Acknowledgements We would like to thank Ehime Beverage Inc. (Ehime, Japan) for supplying juices of C. kawachiensis used in this study. A portion of this work was supported by the Strategic Research and Developmental Project of Ehime Prefecture. This work was supported by the Nagai Memorial Research Scholarship from the Pharmaceutical Society of Japan.