2020 年 26 巻 1 号 p. 101-110
2020 年 26 巻 1 号 p. 101-110
A great deal of attention has been paid to preparation of bioactive peptides from proteins through enzymatic hydrolysis. Enzymatic hydrolysates of diverse food proteins exert various biological activities. In the present study, we prepared and characterized Grifola frondosa protein hydrolysate (GFPH)-selenium chelating complex. Moreover, we assessed the effects of four main independent variables of the chelation conditions and selenium-binding capacity using response surface methodology (RSM). According to the results of RSM, the optimal chelation conditions were as follows: reaction time of 90 min, reaction temperature of 45 °C, pH of 9.0 and the ratio of hydrolysates to sodium selenite (v:v) of 6:4. The content of selenium reached 2 979.45 ± 8.77 µg/g under the optimal conditions. Results of Fourier transform infrared spectroscopy (FTIR) and ultraviolet-visible (UV) spectroscopy indicated the successful incorporation of selenium into GFPH. The analyses of hydroxyl radical scavenging ability and ferric ion reducing antioxidant power (FRAP) were used to evaluate the antioxidant abilities of the newly synthesized chelate. Results showed that the antioxidant activities of GFPH-Se were superior to GFPH. The results of cellular uptake of GFPH-Se chelate in Caco-2 cells suggested that GFPH-Se chelate could be used as a potential source of Se supplement. Taken together, our findings indicated that the by-product of Grifola frondosa was a promising source for peptide-Se bio-products, which could be used as fungus-based functional supplements for humans with Se deficiency.
As an essential trace element for human, animals and some species of microorganism, selenium (Se) has been shown to have several functions (Rayman, 2000) and has received considerable attention over the past few decades. It has been estimated that about one billion people globally may suffer from inadequate intake of Se (Hu et al., 2014). Se deficiency causes several reproductive and obstetric complications, including male and female infertility, miscarriage, foetal growth restriction, gestational diabetes and obstetric cholestasis (Mistry et al., 2012). Bioavailability of Se has become a hot topic in nutrition research. However, just like most trace elements, inorganic Se is toxic to human and animal at high levels (Hamilton, 2004). Therefore, more and more attention has been paid to organic Se due to its greater biological activities and attenuated toxicity (Mao et al., 2016).
In the past several years, many hydrolyzed proteins derived from plant and animal sources have been found to possess biological activities, such as common carp roe (Lian et al., 2013), alfalfa leaf (Xie et al., 2008), porcine plasma (Kong et al., 2013), pea protein (Pownall et al., 2010) and so on. The edible medicinal mushroom, Grifola frondosa (GF, Basidiomycetes, Aphyllopherales, Polyporaceae), is a fungus (Fan et al., 2011), which has been subjected to a large-scale artificial cultivation and increasingly consumed in China and other Asian countries. Fungus contains a variety of bioactive compounds, and one of these main active components is protein. Moreover, many studies have focused on proteins and bioactive peptides produced by GF due to their diverse biological activities, including anti-tumor, antioxidant and anti- HSV and other functions (Dong et al., 2015; Gu et al., 2007; Kodama et al., 2010).
In this study, we aimed to prepare the composites of GFPH chelated with Se, and the FTIR and UV scanning spectroscopy were employed to characterize the GFPH-Se chelate. In order to test antioxidant activities and absorption capacity of the chelate in vitro, we assessed its hydroxyl radical scavenging, FRAP ability and its effect on Se absorption with Caco-2 cells. In summary, our findings provided basic mechanisms on the function and activity of the potential Se supplements.
Materials GF was obtained from the Jingxiang Eco-technology Industry Co., Ltd., Jiangxi, China. The commercial protease, Alcalase, was purchased from Beijing Solarbio Technology Co., Ltd. All of the other chemicals and solvents were of analytical grade, unless otherwise stated.
Preparation of GFPH GF was directly ground into a fine powder by pulverizer and sieved through a 40-mesh filter. Alkaline solution is widely used as an effective and feasible method for protein extraction from plant sources. Therefore, the protein of GF was extracted by alkali-solution and acid-isolation method. The powder of GF was dissolved in deionized water at a substrate concentration of 1:20 (m:v), and then the pH of mixture was adjusted to 11, followed by incubation in water bath at 60 °C for 1.5 h. The pH of supernatant was adjusted to 2.5 after centrifuged at 5 000 rpm for 10 min. The sediment was centrifuged and lyophilized after 8-h static settlement. The pre-processed material was dissolved in deionized water at a substrate concentration of 3% (w/v). Subsequently, the solution was hydrolyzed with alkaline protease under the optimum conditions as follows: the enzyme dose of 5.0% (w/w, defined as enzyme mass/substrate mass × 100%), pH value of 10.0, temperature of 50 °C and enzymatic time of 1 h. After hydrolysis, the alkaline protease was inactivated at 100 °C in water bath for 10 min. The enzymatic hydrolysate was centrifuged at 5 000 rpm for 10 min to remove the insoluble substrates, and the supernatant was collected and stored at 4 °C prior to further analysis.
Formation of GFPH-Se chelate The solution of GFPH was mixed with sodium selenite (0.5 M) at different volume ratios (6:1, 6:2, 6:3, 6:4 and 6:5) and different reaction temperatures (30 °C, 40 °C, 50 °C, 60 °C, 70 °C, 80 °C and 90 °C). To determine the effect of pH on the Se-binding capacity, the reaction pH was maintained at different values (3.0, 4.0, 5.0, 6.0, 7.0, 8.0, 9.0 and 10.0). The reaction was carried out in a shaker at 50 °C for different durations (10, 20, 30, 60, 90 and 120 min). Then absolute ethanol was unceasingly added until the ethanol concentration reached 95% to allow the chelate deposition, and then the solution was centrifuged at 5 000 rpm for 10 min. The obtained GFPH-Se chelate was collected and lyophilized for the content determination of Se and structural characterization of the newly synthesized chelate.
Determination of Se content The Se-binding capacity was determined according to a previously described method (Wesl et al., 2002) with modifications. The content of Se was measured using 3.3'-diaminobenzidine as chromogenic reagent by spectrophotometry.
 |
Where p is the mass concentration of Se from standard curve (µg/mL), V denotes the volume of sample (mL), m represents the mass of sample (g), and N is the ratio of sample volume /total volume.
Experimental design and statistical analysis The optimum combination of GFPH and Se was determined by a central-composite experimental design with four variables. Table 1 lists the independent variables and their levels based on the results of preliminary experiments.
| Variables | −1 | 0 | 1 |
|---|---|---|---|
| Temperature (°C) | 30 | 40 | 50 |
| Time (min) | 30 | 60 | 90 |
| pH | 7 | 8 | 9 |
| Ratio of peptides to sodium selenite(v:v) | 6:03 | 6:04 | 6:05 |
Structural characterization
FTIR The freeze-dried samples (1 mg) were mixed with 100 mg of dried KBr and loaded onto the FTIR. An infrared spectrophotometer (VERTEX 70, BRUKER Co., Ltd., China) was employed to record FTIR spectra ranging from 400 to 4 000 cm−1. The peak signals in the spectra were analyzed using OMNIC 8.2 software (Thermo Nicolet Co., Madison, USA).
UV absorption spectroscopy assay The UV spectra of the GFPH and GFPH-Se chelate were recorded over the wavelength range from 190 to 400 nm by a UV spectrophotometer (UV-2601, RAYLEIGH Instrument Co., Ltd., China) according to a previously described method (Chen et al., 2013). The samples were dissolved in deionized water at a concentration of 0.1 mg/mL and then fluorescently scanned against ultrapure water as blank at room temperature after 30 min.
Determination of antioxidant capacity
Hydroxyl radical scavenging activity The hydroxyl radical (OH) scavenging activity of GFPH and GFPH-Se chelate was assessed by the salicylic acid method (Smirnoff and Cumbes, 1989) with minor modifications. The GFPH or GFPH-Se chelate was dissolved in deionized water at different concentrations (1, 2, 4, 6, 8 and 10 mg/mL). The reaction mixture (4 mL) contained 1 mL H2O2 (8.8 mM), 1 mL FeSO4 (9 mM), 1 mL salicylic and (9 mM) and 1 mL GFPH or GFPH-Se chelate. H2O2 was added into the mixture to initiate the reaction. The reaction mixture was incubated at 37 °C for 30 min and then centrifuged at 5 000 rpm for 10 min. The absorbance of the reaction solution at a wavelength of 510 nm was measured. The scavenging rate was calculated according to the equation as follows:
 |
where A0 is the absorbance for the control (the reaction mixture containing 1 mL double-distilled water), Ax denotes the absorbance for the reaction mixture containing sample solution, and Ax0 represents the absorbance for the background (the reaction mixture without H2O2).
FRAP assay The FRAP of GFPH-Se or GFPH was determined according to a previously described method (Chu et al., 2000) with minor modifications. The GFPH or GFPH-Se chelate was dissolved in deionized water at different concentrations (1, 2, 4, 6, 8 and 10 mg/mL). Subsequently, 2.5 mL GFPH or GFPH-Se chelate was mixed with 2.5 mL phosphate buffer (0.2 M, pH 6.6) and 2.5 mL potassium ferricyanide (1% w/v). The mixture was incubated at 50 °C for 20 min. A total of 2.5 mL trichloroacetic acid solution (10% w/v) was added to the mixture to terminate the reaction. The mixture was then separated into 2.5-mL aliquots, and each aliquot was diluted with 2.5 mL water. Next, 500 µL ferric chloride solution (0.1%, w/v) was added to each diluted aliquot, and the mixture was allowed to stand for 30 min for color development. The absorbance was measured at a wavelength of 700 nm in triplicate.
Effect of GFPH-Se chelate on the cellular uptake of Se
Cell culture Human colon adenocarcinoma cell line Caco-2 provided by Bioengineering Institute of Fuzhou University was used between passage number 20 and 40. Caco-2 cells were maintained in DMEM supplemented with 20% fetal bovine serum, 100 U/mL ampicillin and 100 µg/L streptomycin at 37 °C in a humidified atmosphere containing 5% CO2. For subculture, the confluent cells were enzymatically digested by 0.25% Trypsin-EDTA and split at a ratio of 1:3. At a confluence of 80–90%, cells were seeded into plastic 96-well plates at a density of 1×104 cells/cm2 and incubated for 7 d.
Cytotoxicity test After culture 14 d, the cells were seeded at a density of 2×104 cells/cm2 into 96-well plates. The cytotoxicity test was performed using the MTT assay (Hassan et al., 2017) with minor modifications. Briefly, the culture medium was discarded, and the cells were pre-incubated with DMEM containing GFPH-Se chelate and sodium selenite at 37 °C for 24 h. Subsequently, 20 µL MTT (5 mg/mL in PBS) was added to each well, and the cells were incubated for another 4 h. The solution was carefully aspirated, and the formazan produced by mitochondrial dehydrogenase was dissolved in 150 µL DMSO. Absorbance at 490 nm was determined, and the results were expressed as the proportion of the control.
Cellular uptake experiment The cell layers were washed twice with PBS to completely remove the medium. Cells were incubated with different concentrations of the GFPH-Se chelate (1 mg GFPH-Se chelate involves 2.41 µmol Se) and sodium selenite at 37 °C for 1 h. Then the medium was removed, and the cells were washed with PBS for three times. The cells were placed on ice and incubated with 1 mL cell lysis buffer for 30 min. The cell lysates were transferred into 1.5-mL Eppendorf tubes and centrifuged at 10 000 g for 5 min at 4 °C. The supernatant was collected, and the total protein content and Se content were determined by the bicinchoninic acid (BCA) method and ICP-MS method, respectively. The results of Se uptake were expressed as µg/mg protein.
Statistical analyses All data were presented as means ± standard deviations (SD) of three independent experiments. Statistical analysis was performed using Student's t-test. A P value of < 0.05 was considered statistically significant, and P < 0.01 suggested that the value reached an extremely significant level.
Effects of chelation conditions on Se content The Se content was positively correlated with the pH value (Fig. 1A). When the pH value reached 9.0, the highest Se-binding capacity was detected. The results indicated that GFPH had a stronger ability to chelate Se under the alkaline condition.
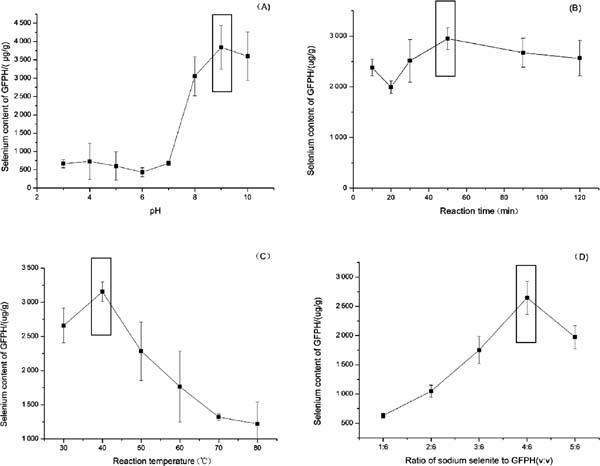
Effects of pH (A), reaction time (B), temperature (C), sodium selenite/GFPH ratio (D) on selenium content.
Fig. 1B shows the correlation between the Se content and reaction time. The curve did not show a significant trend within the tested reaction time. However, the Se-binding capacity was optimum at a reaction time of 50 min. A similar result has been reported that yak casein hydrolysate has the highest Zn-chelating capacity at 6 h, and the Zn-chelating ability is also decreased with extension of reaction time (Wang et al., 2011). The unstable structure of the chelate might contribute to the decrease of Se content.
The effect of reaction temperature on the Se content was also analyzed (Fig. 1C). The highest Se-binding capacity was found when the temperature reached 50 °C.
In order to fabricate the GFPH-Se chelate, we evaluated the effect of the mass ratio of GFPH to Se (Fig. 1D). The Se content was increased when the sodium selenite (0.5 M) was within the range of 1–4 mL, and the best ratio of GFPH to sodium selenite was 6:4 (v:v).
Statistical analysis and model fitting A total of 29 runs were performed to optimize the four individual parameters in the central-composite experimental design. Table 2 shows the experimental factors and the results of Se-binding capacity of GFPH according to the factorial design. The effect of tested variables on the Se content in GFPH-Se chelate could be represented using a second-order polynomial equation as follows:
| Test | A Time (min) | B Temperture (°C) | C pH | D GFPH/Se (v:v) | The content of selenium (µg/g) |
|---|---|---|---|---|---|
| 1 | 60 | 50 | 8 | 3:06 | 2.00×103 |
| 2 | 90 | 40 | 9 | 4:06 | 2.83×103 |
| 3 | 60 | 30 | 7 | 4:06 | 9.56×102 |
| 4 | 90 | 40 | 8 | 3:06 | 1.96×103 |
| 5 | 30 | 50 | 8 | 4:06 | 2.63×103 |
| 6 | 60 | 40 | 9 | 5:06 | 2.74×103 |
| 7 | 60 | 30 | 8 | 5:06 | 1.75×103 |
| 8 | 60 | 50 | 8 | 5:06 | 2.19×103 |
| 9 | 30 | 40 | 7 | 4:06 | 1.56×103 |
| 10 | 60 | 40 | 7 | 5:06 | 2.00×103 |
| 11 | 30 | 40 | 8 | 3:06 | 1.24×103 |
| 12 | 60 | 40 | 8 | 4:06 | 2.48×103 |
| 13 | 60 | 40 | 9 | 3:06 | 1.83×103 |
| 14 | 90 | 40 | 8 | 5:06 | 1.95×103 |
| 15 | 90 | 40 | 7 | 5:06 | 1.02×103 |
| 16 | 60 | 50 | 7 | 4:06 | 1.37×103 |
| 17 | 60 | 40 | 8 | 4:06 | 2.38×103 |
| 18 | 60 | 40 | 8 | 4:06 | 2.49×103 |
| 19 | 90 | 50 | 8 | 4:06 | 2.71×103 |
| 20 | 60 | 30 | 9 | 4:06 | 1.47×103 |
| 21 | 30 | 40 | 9 | 4:06 | 1.84×103 |
| 22 | 30 | 40 | 8 | 5:06 | 1.66×103 |
| 23 | 60 | 40 | 8 | 4:06 | 2.86×103 |
| 24 | 30 | 30 | 8 | 4:06 | 1.07×103 |
| 25 | 60 | 30 | 8 | 3:06 | 1.30×103 |
| 26 | 90 | 30 | 8 | 4:06 | 1.97×103 |
| 27 | 60 | 40 | 7 | 3:06 | 6.58×102 |
| 28 | 60 | 40 | 8 | 4:06 | 2.70×103 |
| 29 | 60 | 50 | 9 | 4:06 | 2.65×103 |
Table 3 presents the ANOVA for response surface quadratic model. The model showed that the correlation coefficient (R2) between the independent variables and dependent variables was 0.9313, indicating that only 6.87% of the total variations were not explained by this model. Moreover, the linear coefficients (A, B, C, D) and quadratic term coefficients (AC, A2, B2, C2, D2) were significant (p < 0.05). The reaction time and pH were key influencers in the process of chelation (p < 0.01). Therefore, the model was adequate for prediction within the range of experimental variables.
| Source | Sum of squares | df | Mean square | F value | p-value |
|---|---|---|---|---|---|
| Model | 1.03×107 | 14 | 7.36×105 | 13.56 | < 0.0001 |
| A-time | 5.02×105 | 1 | 5.02×105 | 9.25 | 0.0088 |
| B-temperature | 2.11×106 | 1 | 2.11×106 | 38.89 | < 0.0001 |
| C-pH | 3.41×106 | 1 | 3.41×106 | 62.89 | < 0.0001 |
| D-GFPH/Se | 6.13×105 | 1 | 6.13×105 | 11.28 | 0.0047 |
| AB | 1.72×105 | 1 | 1.72×105 | 3.16 | 0.0971 |
| AC | 5.80×105 | 1 | 5.80×105 | 10.68 | 0.0056 |
| AD | 47 457.51 | 1 | 47 457.51 | 0.87 | 0.3656 |
| BC | 1.44×105 | 1 | 1.44×105 | 2.66 | 0.1255 |
| BD | 15 468.08 | 1 | 15 468.08 | 0.28 | 0.6018 |
| CD | 8 027.60 | 1 | 8 027.60 | 0.15 | 0.7063 |
| A2 | 3.74×105 | 1 | 3.74×105 | 6.89 | 0.0200 |
| B2 | 5.33×105 | 1 | 5.33×105 | 9.82 | 0.0073 |
| C2 | 1.65×106 | 1 | 1.65×106 | 30.38 | < 0.0001 |
| D2 | 1.38×106 | 1 | 1.38×106 | 25.35 | 0.0002 |
| Residual | 7.60×105 | 14 | 54 282.94 | ||
| Lack of Fit | 7.03×105 | 10 | 70 269.63 | 4.91 | 0.0694 |
| Pure Error | 57 264.80 | 4 | 14 316.20 | ||
| Cor Total | 1.11×107 | 28 | |||
| R2 | 0.9313 | ||||
| Adjusted R2 | 0.8627 |
Visual analysis of the response surface The response surfaces and contour plots were made according to the data of response surface quadratic model by Design-Expert 8.0 (Table 3, Fig. 2). The interaction between the reaction time and pH was significant (p < 0.05), suggesting that there was a complex linear relation among variables and the Se content of GFPH-Se chelate. Moreover, our data showed that the optimal chelation conditions of GFPH binding with Se were as follows: reaction time of 90 min, reaction temperature of 46.89 °C, pH of 9.0 and the ratio of hydrolysate to sodium selenite (v:v) of 6:4. Under these conditions, the Se content was 3 049.83 µg/g.
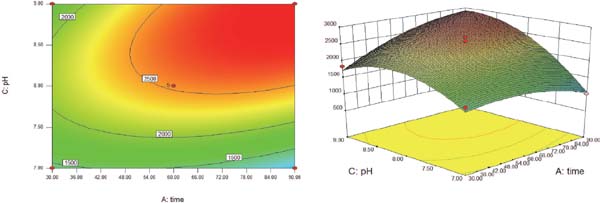
Response surface plot for the effect of cross-interaction between reaction time and pH.
To adapt the experimental operation, the conditions were adjusted to the reaction time of 90 min, reaction temperature of 45 °C, pH of 9.0 and the ratio of GFPH to sodium selenite (v:v) of 6:4. The model was experimentally validated, and the Se content was 2 979.45 ± 8.77 µg/g, indicating that the model was adequate.
Structural characterization
FTIR Changes in the characteristic FTIR absorption peaks of some binding sites in peptides can reflect the interaction of mineral elements with organic ligand groups in the peptide (Zhao et al., 2014). Fig. 3 shows the FTIR spectra of GFPH and GFPH-Se. The results implied that the wave numbers (1 652.49 cm−1 and 1 563.78 cm−1) of the amide I band were shifted to the lower frequencies (1 632.88 cm−1 and 1 576.95 cm−1) after binding with Se, revealing that the absorption of carbonyl groups was induced by the anti-symmetric stretching vibration of C=O and the symmetric stretching vibration of carboxylic acid ions (Xu et al., 2017). Moreover, the band shifting from 3 405.35 cm−1 to 3 429.03 cm−1 was caused by the symmetric stretching vibration of N-H and H bonds of hydration. The oxygen atoms of carboxyl and nitrogen atoms of amino group residues could chelate minerals to form coordinate bonds by donating electron pairs, making the electron cloud density stronger due to the inductive effect or dipole field effect (Chen et al., 2014; Zhao et al., 2014). These findings indicated that the carboxyl oxygen atoms and amino group nitrogen atoms were the interaction sites between GFPH and Se, which was in agreement with the reports by Wang et al., (2014) and Cai et al., (2015). The GFPH bound with Se and formed the GFPH-Se chelate.

The FTIR spectra of GFPH and GFPH-Se chelate.
UV absorption spectroscopy assay The UV spectra of GFPH were obviously different from those of GFPH-Se chelate (Fig. 4). With the addition of Se, the UV spectra of GFPH were significantly altered. As an auxochromic group, the amidogen presented an opposite hypochromic effect at about 197 nm, which was caused by the intervention of Se ions. Furthermore, the changes of strong absorption band also confirmed the binding between the nitrogen atom of amidogen in peptides and Se ions. The spatial structure with the chirality of the chromophores (C=O, −COOH) and auxochromes (−OH, −NH2) of peptides was changed after binding with metal (Armas et al., 2006; Ji et al., 2018) which subsequently induced changes in intensity and red shift in the UV spectra. Both the changes of absorption intensity and the red shift of band suggested chelation of GFPH and Se ion and the formation of a new compound.

The UV absorption spectroscopy of GFPH and GFPH-Se chelate.
Determination of antioxidant capacity
Hydroxyl radical scavenging activity Fig. 5 presents the results of hydroxy radical scavenging analysis. GFPH and GFPH-Se chelate could effectively scavenge hydroxyl radicals. The scavenging activity of GFPH-Se chelate was obviously stronger than that of GFPH when the mass concentration was over 2 mg/mL. Moreover, the hydroxyl radical scavenging rates of both GFPH and GFPH-Se chelate were increased with their concentrations within the range of 1–6 mg/mL. Within a range of high concentrations (6–20 mg/mL), there was still an increasing trend for GFPH-Se, whereas the GFPH presented a decreasing tendency. When the concentration reached 10 mg/mL, the hydroxyl radical scavenging rate of GFPH-Se chelate was up to 97.17 ± 0.13%. This was significantly higher (P < 0.01) than GFPH with a hydroxyl radical scavenging activity of 36.67 ± 3.5%, representing more than twice of that rate. The hydroxyl radical is the most reactive one among oxygen radicals that can damage all types of macromolecules in cells and induce severe damages to cells (Pan et al., 2016). The IC50 value of hydroxyl radical scavenging of GFPH-Se chelate was about 4 mg/mL, indicating that it could reduce or eliminate the damages induced by hydroxyl radicals in foods or biological systems.

The hydroxyl radical scavenging activity of GFPH and GFPH-Se chelate.
FRAP assay The FRAP of GFPH and GFPH-Se chelate was measured at 1–10 mg/mL (Fig. 6). The absorbance was proportionally increased with the antioxidant content. Both the GFPH and GFPH-Se chelate displayed a steadily increasing tendency for reducing power, while the latter was increased more quickly, implying that the FRAP of GFPH-Se chelate was stronger than that of GFPH. When the concentration reached 10 mg/mL, the absorbance of the chelate was 1.42, which was the double value compared with the hydrolysate. Antioxidants may exert their protective effects by reducing metal ions, therefore influencing the oxidative stress caused by these metal ions. This reducing ability of antioxidants can be assessed according to the FRAP method (Dolatabadi et al., 2014). These findings suggested that GFPH and GFPH-Se chelate were capable of donating electrons, and they could react with free radicals or terminate chain reactions, whereas metal chelation reduced electron transfer from peptides after binding with Se.

The FRAP activity of GFPH and GFPH-Se chelate.
The analyses of hydroxyl radical scavenging ability and FRAP indicated that transferring of hydrogen atom and electron donation were the main mechanisms during antioxidant process (Ravichandran et al., 2013). It has been reported that mineral elements with protein hydrolysate or peptides exhibit stronger antioxidant activity (Huang et al., 2015; Megías et al., 2008). Meanwhile, GFPH-Se chelate possessed stronger antioxidant activity and FRAP activity compared with GFPH.
Se bioavailability in human intestinal Caco-2 cell line The MTT assay was performed to evaluate the effects of GFPH-Se chelate and sodium selenite on cytotoxicity of Caco- 2 cells. Fig. 7A shows that both GFPH-Se chelate and sodium selenite inhibited the proliferation of Caco-2 cells, and Caco-2 cells were not inactivated at a Se concentration of 8 µmol/L. For the uptake assay, Caco-2 cells were pre-incubated with GFPH-Se chelate at different concentrations. Changes of the intracellular Se content were determined by ICP-MS. Within the range of 1–6 µmol/L, the effect of GFPH-Se chelate on Se uptake efficiency of Caco-2 cells was dose-dependently increased, and then it was decreased when the Se concentration reached 8 µmol/L (Fig. 7B). However, the inorganic Se absorption was firstly increased, and then decreased beyond 4 µmol/L sodium selenite. A large number of studies have demonstrated that organic Se presents a better ability of Se absorption compared with inorganic Se (Todd et al., 2012). Too much inorganic Se may inhibit the absorption of Se. Moreover, the Se content in Caco-2 cells was 0.52 µg/mg protein when the Se concentration reached 6 µmol/L, which was significantly higher (p < 0.01) compared with sodium selenite. Therefore, the GFPH-Se chelate could be a good choice for the Se supplement.
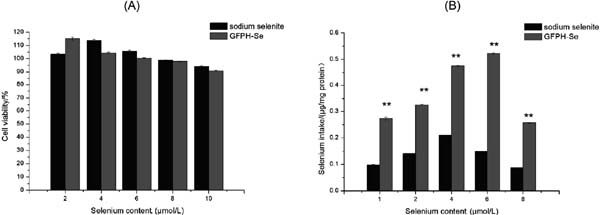
Cellular uptake of GFPH-Se chelate and sodium selenite in Caco-2 model. (A) MTT assay was performed to determine the cytotoxic effect of GFPH-Se chelate and sodium selenite. (B) Cellular uptake of GFPH-Se chelate and sodium selenite by ICP-MS. All the samples were determined three times, and the data were reported as mean ± SD.
In summary, we investigated the optimum conditions of GFPH-Se chelate via RSM in the present study. The results of FTIR and UV absorption spectroscopy assay revealed that the carboxyl oxygen atoms and amino group nitrogen atoms were the interaction sites between GFPH and Se. In addition, the chelation reaction between GFPH and Se ions happened and generated a new chelate. Radical scavenging activity analysis showed that GFPH-Se chelate had stronger scavenging activity of hydroxyl radicals and FRAP activity compared with GFPH. The cellular uptake of GFPH-Se chelate and sodium selenite in Caco-2 cells was also investigated. Our findings indicated that the GFPH-Se chelate could be applied in the commercial food production as a new dietary nutrient, serving as a novel type of Se supplement with antioxidant properties.
Acknowledgments This research was supported by National Natural Science Foundation of China (No. 31501432), the Young and Middle-aged Teachers Education and Scientific Research Project of the Fujian Province (No. JZ180907) and the General Research Program of the Liming Vocational University (No. LZ2019115).
selenium
SDstandard deviations
GFPHGrifola frondosa protein hydrolysate
FTIRFourier transform infrared spectroscopy
RSMresponse surface methodology