2020 年 26 巻 1 号 p. 53-58
2020 年 26 巻 1 号 p. 53-58
This study evaluated the survival of Listeria monocytogenes in kimchi using predictive models. Kimchi inoculated with L. monocytogenes was stored at 4, 15, and 25 °C, and the cells were enumerated on PALCAM agar. The Baranyi model was fitted to L. monocytogenes counts to calculate lag phase duration, maximum specific growth rate (µmax; Log CFU/g/h) and death rate (DR; Log CFU/g/h). A polynomial equation was fitted to the kinetic parameters, followed by development of a dynamic model. While L. monocytogenes showed sparse growth at 4 °C, obvious growth was observed at 0.041 (15 °C) and 0.185 (25 °C) µmax. After 96 (4 °C), 60 (15 °C), and 24 (25 °C) h, L. monocytogenes cells started to decrease at DRs of 0.023, 0.039, and 0.144, respectively, with an RMSE of 0.370, indicating appropriate performance of the models. These results indicate that L. monocytogenes can survive in kimchi, even when it is fermented sufficiently.
Kimchi is a Korean dish made from fermented vegetables such as napa cabbage and radish, various spices including garlic, ginger, red pepper powder, and other ingredients (Cheigh et al., 1994). It contains various nutrients such as dietary fiber, vitamins, carotene, and minerals (Cheigh et al., 1994). Moreover, it exhibits functional effects such as anticancer and antiobesity activities, improvement of cholesterol level, and probiotic characteristics (Park et al., 2014); therefore, fermented kimchi is added to other food products as an ingredient (Yu et al., 2017; Yoon et al., 2018). In addition, in kimchi, lactic acid bacteria (LAB) produce lactic acid and bacteriocin during fermentation, creating an antimicrobial environment (Kwon et al., 2002; Kim et al., 2015 Li et al., 2015). Hence, kimchi has been considered to be a microbiologically safe food (Choi et al., 2018b). However, foodborne outbreaks involving kimchi from school meal services and recalls have occurred in South Korea and the United States because of foodborne pathogen contamination in kimchi, especially Listeria monocytogenes in napa cabbage (Food Safety News, 2011; NSP News Agency, 2011; KCDC, 2014; Kim et al., 2014).
L. monocytogenes contamination in napa cabbage kimchi should be considered a serious threat, as napa kimchi is the most popular kimchi, and the pathogen is the causative agent of listeriosis, which has a mortality rate of approximately 20%–30% (Muhterem-Uyar et al., 2015; Ha and Ju, 2016; Lee et al., 2017). Hence, heightened awareness of the risk of L. monocytogenes in napa cabbage kimchi is needed (Oh et al., 2018). Fermented kimchi is usually consumed without additional heating; therefore, L. monocytogenes in contaminated kimchi can survive the fermentation process, and may cause serious problems such as sepsis, fetal infections, and even death (CDC, 2013). Thus, it is vital to elucidate the survival kinetics of L. monocytogenes in kimchi using predictive models. The objective of this study was to develop predictive models and to kinetically evaluate the survival of L. monocytogenes in napa cabbage kimchi using these models.
Bacterial inoculum preparation Nine L. monocytogenes strains (NCCP10943, NCCP10920, NCCP10811, NCCP10810, NCCP10809, NCCP10808, NCCP10807, NCCP10806, and NCCP10805) were cultured in 10 mL of tryptic soy broth supplemented with 0.6% yeast extract (TSBYE; BD, Sparks, USA) at 30 °C for 24 h. The 1-mL aliquots of cultures were transferred to 10 mL of fresh TSBYE and cultured at 30 °C for 24 h. The subcultures of nine L. monocytogenes strains were mixed, and then centrifuged (1 912 g, 4 °C, 15 min). The cell pellets were washed twice with phosphate-buffered saline (PBS) (0.2 g KCl, 1.5 g Na2HPO4·7H2O, 8.0 g NaCl, and 0.2 g KH2PO4, in 1 L of distilled water; pH 7.4) and diluted with PBS to prepare the inoculum at 6 Log CFU/mL.
Kimchi preparation and inoculation For napa cabbage kimchi, the raw ingredients, including napa cabbage, white radish, spring onion, crushed garlic, crushed ginger, powdered red pepper, and sugar were purchased from grocery stores. Napa cabbage kimchi was prepared with these ingredients according to the recipes by RDA (2008) and Korean Traditional Knowledge Portal (2015) with slight modifications. After washing the white radish and spring onion with distilled water to remove any dirt, they were cut into 5-cm long pieces. Napa cabbages were salted in 10% sodium chloride water for 3 h, rinsed with distilled water, and cut into 4-cm long pieces with a knife. Salted napa cabbage pieces (1 kg) were mixed thoroughly with the prepared ingredients (172 g julienned white radish, 16 g spring onion, 24 g crushed garlic, 3.2 g crushed ginger, 22 g powdered red pepper, and 2 g sugar). One kilogram of the prepared napa cabbage kimchi was placed in a zippered bag (30 × 45 cm) for storage and fermentation. The samples were inoculated with 20 mL of inoculum and massaged thoroughly, and the zippered bags were placed into an airtight plastic container, followed by incubation at 4, 15, and 25 °C for 336, 240, and 240 h, respectively, for fermentation.
Microbiological analyses Kimchi samples were analyzed at the appropriate time intervals during fermentation for microbiological analysis. Fifty grams of kimchi samples were transferred aseptically into filter sample bags (3M; St. Paul, USA) containing 100 mL of 0.1% buffered peptone water (BPW), followed by pummeling (BagMixer; Interscience, St. Nom, France) for 60 s. After the homogenates were diluted with BPW, 0.1 mL aliquots of the diluents were plated on PALCAM agar (BD) and de Man Rogosa Sharpe (MRS) agar (BD) to enumerate the cell counts of L. monocytogenes and LAB, respectively. PALCAM and MRS agar plates were incubated at 30 °C for 24 h and 37 °C for 48 h, respectively. The homogenates were subjected to pH measurement using a pH meter (Thermo Electron Corporation, Waltham, USA). Each experiment was repeated twice with two samples per trial (n=4).
Kinetic model development To develop a primary model capable of describing the kinetic behavior of L. monocytogenes, the Baranyi model (Baranyi and Roberts, 1994) was fitted to the data of L. monocytogenes cell counts recovered from kimchi to calculate the maximum specific growth rate (µmax; Log CFU/g/h) and lag phase duration (LPD; h) for the growth phase, death rate (DR; Log CFU/g/h) for the death phase, initial level (N0; Log CFU/g), and final level (Nmax; Log CFU/g) using a DMFit (Institute of Food Research, Norwich, UK).
 |
Where Nt (Log CFU/g) is the bacterial cell count at any time t, and At is the adjustment function. Because L. monocytogenes showed growth and death phases in the napa cabbage kimchi, initiation time to death (ITD) was estimated from the fitted curves. The secondary model was developed to describe the effect of storage temperature on the kinetic parameters. The polynomial equation was fitted to µmax, DR, and ITD with SigmaPlot 10.0 (Systat Software, San Jose, USA).
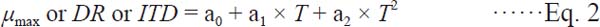 |
Where ai are coefficients, representing any number from 0 to 2, and T is the storage temperature (°C). To validate the developed models, RMSE, Af, and Bf were calculated by comparing the predicted L. monocytogenes cell counts and the observed cell counts, obtained from the other study at 10 °C and 20 °C performed in our laboratory (Ross, 1996).
 |
Where n is the number of data points.
Furthermore, a dynamic model was developed using the developed secondary models and the equation described by Baranyi et al. (1995) to predict L. monocytogenes growth in kimchi under different storage temperatures (5–25 °C) during fermentation. The kinetic parameters calculated at the given temperatures were used to estimate L. monocytogenes cell counts using the primary model, and the predicted values for L. monocytogenes cell counts were compared to the observed values.
L. monocytogenes showed both a growth phase and a death phase in napa cabbage kimchi during fermentation (Fig. 1). To calculate the parameters related to the fate of L. monocytogenes, the Baranyi model was fitted to the data of L. monocytogenes cell counts recovered from napa cabbage kimchi during the growth and death phases. The R2 values of the fitting ranged from 0.642 to 0.934 (Table 1), indicating that the goodness of fit for the primary model was appropriate. In the growth phase, N0 were 3.7–3.9 Log CFU/g regardless of the storage temperature, and Nmax were 4.0, 5.4, and 6.2 Log CFU/g at 4, 15, and 25 °C, respectively (data not shown). LPD at 4 °C was not calculated, as sparse growth of L. monocytogenes was observed during fermentation, and the LPD values at 15 and 25 °C were 12.86 and 9.44 h, respectively (Table 1). In the growth phase, µmax values were 0.001, 0.041, and 0.185 Log CFU/g/h at 4, 15, and 25 °C, respectively (Table 1). These results indicate that L. monocytogenes cell counts were not dramatically changed at 4 °C; however, the cell counts began increasing rapidly immediately after short LPD at 15 and 25 °C, which are typical temperatures for early fermentation for 12– 24 h.

Observed Listeria monocytogenes cell counts and the fitted line by the Baranyi model, and lactic acid bacteria cell counts and pH in napa cabbage kimchi during fermentation at 4 °C (A), 15 °C(B), and 25 °C (C).
| Growth phase | ITD3) (h) | Death phase | R2 | ||
|---|---|---|---|---|---|
| Storage temperature (°C) | LPD1) (h) | µmax2) (Log CFU/g/h) |
DR4) (Log CFU/g/h) |
||
| 4 | - | 0.001±0.000 | 96±0 | −0.023±0.032 | 0.642 |
| 15 | 12.86±8.22 | 0.041±0.001 | 60±17 | −0.039±0.008 | 0.949 |
| 25 | 9.44±1.60 | 0.185±0.000 | 24±0 | −0.144±0.012 | 0.934 |
After reaching the maximum cell counts, L. monocytogenes cell counts in napa cabbage kimchi started to decrease at DRs of –0.023 Log CFU/g/h (4 °C), –0.039 Log CFU/g/h (15 °C), and –0.144 Log CFU/g/h (25 °C) (Table 1). The ITDs were 96, 60, and 24 h after the fermentation started at 4, 15, and 25 °C, respectively (Table 1). According to Kim et al. (2006), some kimchi distributed to institutions is fermented at room temperature for 12 h and 3 to 4 d in summer and winter, respectively, prior to low-temperature storage, suggesting that L. monocytogenes can increase during initial storage and gradually decrease thereafter. This result indicates that if L. monocytogenes is not eliminated completely from the raw ingredients used for kimchi, it can survive during distribution.
During fermentation at 4 °C for 336 h, there was no growth of LAB, and there was minimal change in pH. In contrast, at 15 °C and 25 °C, LAB cell counts increased in the early phase of fermentation, with a subsequent decrease in L. monocytogenes cell counts. However, L. monocytogenes cell counts remained constant after the 1–2 Log CFU/g reduction, whereas Escherichia coli and Salmonella cell counts are generally reduced to below the detection limit after kimchi is fermented sufficiently (Choi et al., 2018a, b). The acid resistance of L. monocytogenes in kimchi may be related to the glutamate decarboxylase system. In this system, glutamate is internalized and converted to gamma-aminobutyric acid (GABA), consuming an intracellular proton. GABA is subsequently exchanged for another extracellular glutamate via a membrane-located antiporter (Cotter et al., 2001). This prevents the decrease in intracellular pH of L. monocytogenes. As a result of this property, the pathogen becomes resistant to the low pH condition of kimchi. GABA is also produced by LAB in kimchi through the glutamate decarboxylase system (Ueno, 2000; Cho et al., 2007; Lim et al., 2017). This can also facilitate the survival of LAB at low pH conditions. In 2011, napa cabbage kimchi was recalled due to L. monocytogenes contamination (Food Safety News, 2011). This recall could be related to the ability of L. monocytogenes to be resistant to the low pH of kimchi.
LAB increased as a function of storage (fermentation) temperature, which affected the decrease in L. monocytogenes; thus, storage temperature was considered as the main factor in the kinetic behavior of L. monocytogenes. To calculate the effect of storage temperature on kinetic parameters (µmax, LPD, and ITD), the secondary models were developed with an R2 of 0.933 to 1.000 of (Table 2). This result showed that the kinetic parameters were influenced by fermentation temperature.
| Kinetic parameter | Secondary model | R2 |
|---|---|---|
| µmax1) | 0.0169−0.0061 − Temp4) + 0.0005 × Temp2 | 1.000 |
| DR2) | −0.0436 + 0.0068 × Temp −0.0004 × Temp2 | 0.933 |
| ITD3) | 108.1558−2.9766 × Temp−0.0156 × Temp2 | 0.947 |
To evaluate the performance of the developed models, root mean square error (RMSE), accuracy factor (Af), and bias factor (Bf) were calculated. The values of 1 for Af and Bf indicate perfect agreement between predictions and observations. The closer the RMSE value is to 0, the better the performance of the model (Ross, 1996). In this study, Af and Bf were 1.04 and 1.05, respectively, and RMSE value was 0.370, indicating that the performance of the developed models was appropriate.
To describe the kinetic behavior of L. monocytogenes in napa cabbage kimchi under various temperature conditions, the dynamic model was developed (Fig. 2). The dynamic model showed that even at changing fermentation temperatures (45, 15, and 25 °C), L. monocytogenes cell counts increased from 3.7 to 5.2 Log CFU/g, and gradually decreased to 2.7 Log CFU/g during fermentation for 120 h (Fig. 2). The difference between the observed and predicted values was on average 0.5 Log CFU/g, indicating that the developed dynamic model showed suitable performance.

Dynamic model to describe Listeria monocytogenes cell counts in napa cabbage kimchi during fermentation at changing storage temperatures (5–25 °C); symbol: observed values, solid line: predicted value, and dotted line: storage temperature.
In conclusion, the developed mathematical models could appropriately describe the survival kinetics of L. monocytogenes in napa cabbage kimchi during fermentation. The developed predictive models showed that L. monocytogenes cell counts were constant during 4 °C fermentation; however, the cell counts increased at the start of fermentation at 15 and 25 °C and then decreased after LAB cell counts reached a maximum. During fermentation at room temperature as the initial storage condition, L. monocytogenes cell numbers in kimchi can increase, and the cells can survive at refrigeration temperatures even as they decrease with the passage of time. Therefore, even when kimchi is fermented sufficiently, L. monocytogenes can survive in napa cabbage kimchi, indicating the importance of decontaminating the raw ingredients before application to fermentation.
Acknowledgements This research was supported by the Main Research Program E0192101-01 of the Korea Research Food Institute funded by the Ministry of Science, ICT, and Future Planning.