2020 Volume 26 Issue 4 Pages 487-494
2020 Volume 26 Issue 4 Pages 487-494
In our previous work, we have proved that diosgenin (DS) can promote the Tuber polysaccharide (TP) production of Tuber melanosporum in the liquid-state fermentation process. In the present study, we confirm that DS can increase the TP production in the solid-state fermentation of T. melanosporum. The orthogonal design and non-linear regression analysis revealed that the optimum medium composition was as follows (g/L): oat 60, corn hull 15.2, fructose 1.8, sucrose 1.2, peptone 0.48, yeast extract 0.72, MgSO4·7H2O 0.06, MnSO4 0.12, KH2PO4·H2O 0.09, VB2 0.01 and DS 0.4 at a water-material ratio of 0.85. The optimum operational condition included inoculation volume of 19.2 mL, culture time of 12.9 d and culture temperature of 26.6 °C. Under the optimum medium and operational conditions, the maximum TP production reached 115.6 mg/g dry substrate. Collectively, our findings provided a strategy to increase bioactive ingredients of traditional food.
As an exophytic edible fungus, Tuber melanosporum and a relationship with trees symbiosis form truffle (Frank, 2005). Due to its high contents of proteins, polysaccharides, fats, carbohydrates, ash, phenolics and other organic molecules, truffle has been used as a source of food, nutrition, and treatment for many years (Khalifa, et al., 2019). Moreover, truffle is known as one of three greatest delicacies in western countries (Mello, et al., 2006). Therefore, truffle has both high food value and economic value (Patel, et al., 2017).
Polysaccharides are one of the most important ingredients in truffles. Truffle's polysaccharide (TP) has non-toxic side effects on normal cells and possesses a variety of biological activities, such as antioxidant (Zhao, et al., 2015), immunity-regulating (Schillaci, et al., 2017), anti-inflammation (Chu, et al., 2019), anti-tumor (Zhao, et al., 2012), blood sugar-lowering, and blood lipid-reducing properties (Devshri, et al., 2019). In recent years, due to its unique biological activities, people have paid great attention to TP (Luo, et al., 2011). Moreover, TP also plays a critical role in the development of healthy food and medicine.
The mushroom polysaccharide can be obtained from mushroom fruit, liquid-state fermentation (LSF) and solid-state fermentation (SSF). However, as a simple process, SSF is cost-effective, which is characterized by reduced levels of catabolite repression and end-product suppression, and it generates less amount of wastewater, permits higher product recovery and yields an excellent product (Hölker and Jürgen, 2005). Therefore, a great deal of attention has been paid to SSF for the production of primary and secondary metabolites. Many edible filamentous fungi and yeast synthesize polysaccharide (Pandey, et al., 2000). Camelini et al. have used wheat as a substrate to obtain polysaccharide via SSF of Agaricus subrufescens (Li, et al., 2006). Chen et al. have prepared polysaccharides from Inonotus hispidus via SSF, and optimized the extraction condition of I. hispidus's polysaccharides using an ultrasonic-assisted extraction method (Chen, et al., 2011).
The most critical issue is how to increase the yield of polysaccharides in the LSF or SSF process. The general strategies include mutagenic breeding, optimization of medium components and cultivation conditions, and metabolism control (Wang, et al., 2015). A few studies have focused on increasing the yield of polysaccharides using herbs or their extracts (Zhang, et al., 2013). In the previous study, we have found that diosgenin (DS) can increase the exo-polysaccharide (EPS) production in the LSF process of T. melanosporum (Zhang, et al., 2019). However, it remains unclear whether DS can increase the TP yield in the SSF process of T. melanosporum.
Oat (Avena sativa) is suitable for human consumption as o atmeal and rolled oats. However, its consumption and global yield are significantly lower than those of the staple crops, such as wheat, maize, rice and barley. One possibility can be attributed to that the processing of oat in our country is still at the primary stage, and most of the processed oat products sold in the domestic market are made from raw materials or primary products. Nevertheless, oats possess higher contents of certain nutrients and phytochemicals, such as essential amino acids and unsaturated fatty acids, vitamins, minerals (Singh, et al., 2013), phenolics, cellulose, arabinoxylan and β-glucan, compared with other cereals (Menon, et al., 2016). Oat consumption can lower blood cholesterol, and ameliorates insulin sensitivity and post-prandial glycaemic control (Bao, et al., 2014; Ho, et al., 2016). Therefore, the development of oat products has a broad market prospect. In the present study, we aimed to further improve the TP content of oat in the SSF process of T. melanosporum. Moreover, we also attempted to provide a health food with better flavor for diabetic patients and people with impaired glucose tolerance.
Raw materials and microorganism The T. melanosporum was kindly provided by Prof. Hailong Li (Zhejiang Subtropical Crops Institute; China). DS was obtained from Shanxi Zhongxin Bio-Science and Technology Co., Ltd. All grains were supplied by local supermarkets. All chemicals and reagents were of analytical or reagent grade and purchased from Sinopharm Chemical Reagent Co., Ltd., Shanghai, China.
Preparation of the liquid seed T. melanosporum seed culture was inoculated into potato dextrose agar (PDA) slant and incubated at 28 °C for 5 d, followed by the storage at 4 °C for approximately 2 months. The liquid seed medium composed of (g/L) glucose 35, peptone 5, yeast extract 2.5, KH2PO4·H2O 1, and MgSO4·7H2O 0.5 was loaded into 250-mL flasks. After inoculation, these flasks were placed on a rotary shaker (120 rpm), followed by incubation at 25 °C for 5 d. The liquid culture was the seed of SSF.
The preparation of SSF production of T. melanosporum The SSF was carried out in the stainless steel shallow dishes (200×200×20 mm). Each dish was loaded with SSF medium. After sterilization at 120 °C for 60 min, dishes were sealed with preservation film to retain moisture content of medium. After cooling to room temperature, 20 mL of seed liquid culture was then inoculated into the dishes, followed by incubation in a growth incubator at 25 °C for 7 d. Basal SSF medium consisted of 60 g oat (or other grains), 15 g corn hull and 60 mL H2O. After incubation for 7 d, the solid substrate was dried at 70 °C to constant weight.
Effects of different grains on the TP production Briefly, the effects of various grains on the TP production were explored using SSF medium consisting of 60 g grains (rice, wheat, rice, buckwheat, broomcorn or corn), 15 g corn hull, 0.45 g DS and 60 mL H2O. Medium in the absence of DS was used as the control. The medium was sterilized at 121 °C for 60 min. After 20 mL liquid seed culture was inoculated, these dishes were incubated at 25 °C for 7 d. Subsequently, the substrate was dried at 70 °C to constant weight, and the TP yield was examined.
The synergic effect of medium components and DS on the TP production In the preliminary test and LSF tests, 8 factors, including fructose, sucrose, peptone, yeast extract, MgSO4, MnSO4, KH2PO4 and VB2, were found to significantly affect the yield of polysaccharide. The influence of seven factors on the yield of polysaccharide can be determined by the single factor design. However, this process greatly reduces efficiency when there is an interdependency of factors or when it is impractical to isolate and test each variable individually. Orthogonal design is much more efficient in determining the optimal combination of factors than the single factor design. Orthogonal design may save large and costly experiments when many factors are involved (Rao, et al., 2008). Orthogonal arrays have been successfully applied to address the problem of determining adequate combinations of factors in biological and biotechnological processes (Rao, et al., 2008; Assemi, et al., 2012; Sedghi, et al., 2014; Vasilev et al., 2014). Therefore, the L50 (59) orthogonal experiment was designed to analyze the synergic effects between these factors and DS on production of extracellular polymeric substance (EPS). These above-mentioned nine factors and five levels of each factor were shown in Table 1. The experimental scheme was designed using the orthogonal design assistant (version VII3.1, China). Each test was performed for three times, and the results were reported as mean.
| Factor | Level (g/60g oat) | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Fructose | 0.6 | 1.2 | 1.8 | 2.4 | 3 |
| Sucrose | 0.6 | 1.2 | 1.8 | 2.4 | 3 |
| Peptone | 0.24 | 0.48 | 0.72 | 0.96 | 1.2 |
| Yeast extract | 0.24 | 0.48 | 0.72 | 0.96 | 1.2 |
| MgSO4 | 0.03 | 0.06 | 0.09 | 0.12 | 0.15 |
| MnSO4 | 0.03 | 0.06 | 0.09 | 0.12 | 0.15 |
| KH2PO4 | 0.03 | 0.06 | 0.09 | 0.12 | 0.15 |
| VB2 | 0.01 | 0.02 | 0.03 | 0.04 | 0.05 |
| DS | 0.15 | 0.3 | 0.45 | 0.6 | 0.75 |
Effect of the synergic action between culture condition and DS on the TP production The conditions influencing TP production in the SSF process include culture temperature, culture time, inoculation volume, air-permeability and a water-material ratio. Five L25 (52) orthogonal experiments were designed to assess the effect of synergic action between these factors and DS on the TP production. The used basic fermentation medium consisted of 60 g oat, 1.8 g fructose, 1.2 g sucrose, 0.48 g peptone, 0.72 g yeast extract, 0.06 g MgSO4·7H2O, 0.12 g MnSO4, 0.09 g KH2PO4·H2O, 0.01 g VB2, and 0.45 g DS. These above-mentioned five factors and five levels of each factor and DS were shown in Table 2. The experimental scheme was designed using the orthogonal design assistant. Each test was performed for three times, and the results were reported as mean.
| Factor | Level (g/60g oat) | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Inoculation volume (mL) | 5 | 10 | 15 | 20 | 25 |
| Culture time (d) | 5 | 6 | 7 | 8 | 9 |
| Culture temperature (°C) | 15 | 20 | 25 | 30 | 35 |
| Corn bran (g) | 9 | 12 | 15 | 18 | 21 |
| Water volume (mL) | 50 | 55 | 60 | 65 | 70 |
| DS (g) | 0.15 | 0.30 | 0.45 | 0.60 | 0.75 |
Measurement of TP The dried SSF substrate was ground into a fine powder. Briefly, 5 g substrate powder was isolated with 25 mL H2O in a water bath at 60 °C for 4 h. After extraction, the extract was hydrolyzed by 1 mL amylase at 80 °C for 1 h and further hydrolyzed by 1 mL glycosylase at 60 °C for 1 h. The hydrolysate was centrifuged at 10 000 × g for 15 min, and then 5 mL supernatant was precipitated with 20 mL 95 % ethanol (v/v) at 4 °C for 24 h. Subsequently, the crude EPS was centrifuged at 10 000 × g for 10 min, and the residuals were resuspended in 1 M NaOH, followed by determination of TP content. The TP content was calculated with phenol-sulfuric acid colorimetric method, and D-glucose was used as the standard (Tang and Huang, 2018). All assays were conducted in triplicate, and data were reported as means ± standard error. One-way analysis of variance (ANOVA) was employed to statistically analyze the data, followed by Duncan's multiple range test (DMRT). A p < 0.05 was regarded as statistically significant. Excel software and SPSS software 17.0 (IBM, Ammonst City, USA) were employed to fit all data.
Optimization of culture conditions and medium components is usually carried out in the Erlenmeyer flask. The narrow neck of Erlenmeyer flask impacts the aeration in the SSF, which is not suitable to study of SSF. Therefore, plastic bowls with a diameter of 120 mm and a depth of 90 mm have been used to assess SSF in the previous study (Xu, et al., 2019). However, plastic bowl is too deep, leading to airflow obstruction. In the present study, the medium components and culture conditions were optimized using the stainless steel shallow dish (200×200×20 mm). Certain factors may play critical roles in the SSF process to enhance the TP production, including medium component, amount of DS added, inoculum volume, air-permeability, initial pH, incubation time, and incubation temperature. Initial pH or pH control plays a key role in the submerged fermentation process to elevate the production of fungal polysaccharides (Oliveira, et al., 1994; Fang, et al., 2002). However, a solid medium has a strong buffer capacity of pH. The pH adjustment requires additional acids or bases, negatively affecting the food flavor. In the present study, a natural pH of the solid medium was not adjusted, and we tested the effects of medium components, water-material ratio, inoculum volume, medium weight, incubation time, and incubation temperature on TP production.
Effects of various grains on the yield of TP Although main components of various grains were starch, protein, fatty and so on, their bioactive components and the contents of these components were different. These bioactive components might affect the synthesis and secretion of TP, and finally cause differences in TP production. Therefore, we first compared the effects of six grains on the TP production in the presence or absence of DS. Fig. 1. exhibits that when rice, buckwheat, oat and broomcorn were used as substrate, DS could significantly increase the TP production. Among these substrates, when oat was used as a substrate, the TP production increased by DS was extremely significant, and the TP yield was the highest, reaching 18.79 mg/g dry substrate. Therefore, we used oat as a fermentation substrate. Similar results have been obtained by Harlow et al. (2017) and Marklinder and Lonner (1992).

Effects of various substrates on the polysaccharide production of T. melanosporum.
The synergic effects of medium components and DS on the TP production Metabolite productions of SSF can be significantly affected by carbon sources, nitrogen sources, inorganic salts and growth factors (Pandey, et al., 2000; Liu and Tang, 2010; Gessesse and Mamo, 1999). According to the preliminary test (data not shown) and results of LSF (Zhang, et al., 2019), fructose (F1), sucrose (F2), peptone (F3), yeast extract (F4), MgSO4 (F5), MnSO4 (F6), KH2PO4 (F7), VB2 (F8) and DS (F9) were identified as crucial factors to affect TP production. Therefore, their synergic effects on TP production were studied by using nine factors as independent variables and the TP production as the dependent variable. Additional file 1: Table 1 summarized the running result of the orthogonal design. The R2 (0.869) and p value of orthogonal design (0.046) were less than 0.05 (in Table 3), suggesting that the model effect was significant, and the orthogonal test results were reliable and statistically significant. The p value of fructose and DS coefficients was not higher than 0.01 (in Table 3), indicating that the two components had an extremely significant effect on TP production. The p value of KH2PO4 was less than 0.05 (in Table 3), indicating that KH2PO4 had a dramatic effect on TP production. A bigger t value of a parameter in Table 3 reflects a stronger impact on the TP production. According to the t values of all factors, we could deduce that the effect of each factor on TP production could be ranked as follows: F9> F1> F7> F2> F4> F6> F8> F3> F5, namely, DS> fructose> KH2PO4> sucrose> yeast extract> MnSO4> VB2> peptone> MgSO4. According to the magnitude range analysis in Table 3, the optimal medium combination could be F1(3) F2(2) F3(2) F4(3) F5(2) F6(4) F7(3) F8(1) F9(3). In other words, the optimum medium composition included 60 g oat, 1.8 g fructose, 1.2 g sucrose, 0.48 g peptone, 0.72 g yeast extract, 0.06 g MgSO4·7H2O, 0.12 g MnSO4, 0.09 g KH2PO4·H2O, 0.01 g VB2, and 0.45 g DS.
| Run | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 |
|---|---|---|---|---|---|---|---|---|---|
| Analysis of variance | |||||||||
| t | 5.259 | 2.821 | 0.476 | 1.595 | 0.101 | 1.532 | 3.420 | 0.820 | 5.593 |
| p | 0.010 | 0.069 | 0.753 | 0.234 | 0.980 | 0.250 | 0.040 | 0.535 | 0.008 |
| Model: R2 = 0.869; adj-R2 = 0.507; p = 0.046. | |||||||||
| Magnitude Range | |||||||||
| k1 | 27.57 | 26.66 | 25.48 | 24.46 | 25.70 | 24.64 | 24.67 | 27.44 | 24.29 |
| k2 | 24.06 | 27.30 | 26.98 | 25.16 | 26.30 | 25.92 | 27.50 | 25.11 | 25.44 |
| k3 | 29.17 | 24.44 | 25.20 | 28.01 | 25.37 | 26.98 | 28.35 | 25.44 | 30.20 |
| k4 | 23.61 | 27.26 | 26.06 | 25.89 | 25.92 | 27.2 | 24.12 | 25.90 | 24.19 |
| k5 | 24.66 | 23.42 | 25.34 | 25.55 | 25.79 | 24.33 | 24.43 | 25.19 | 24.96 |
Synergic effect between DS and different operational conditions on EPS Operational conditions, including incubation time, incubation temperature, inoculation volume, medium air-permeability and water-material ratio, have a significant effect on the TP production in the SSF process (Tang, et al., 2008; Ikasari, et al., 1999; Smits, et al., 1998). In the process of SSF, the medium air-permeability was adjusted by some grain brans, such as corn hull, wheat bran or rice hull. Because corn hull mainly contains protein, cellulose and a small amount of starch, we selected corn hull to increase the medium air-permeability. To study the synergic effect between DS and different operational conditions on TP production, five L25 (52) orthogonal designs were performed. Additional file 2: Table 2 shows the designs and running results of five L25 (52) orthogonal experiments. To accurately disclose the synergic effects between DS and operational conditions, the data in Additional file 2: Table 2 were analyzed by non-linear regression with following equation:
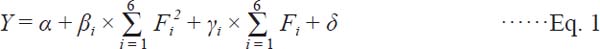 |
Where F1–F6 are expressed as DS amount, inoculation volume, incubation time, incubation temperature, corn hull and water-material ratio, respectively; Y is the TP production; α is the constant; βi is the co-efficient of Fi2; γi is the co-efficient of Fi; and δ is the residual.
Table 4 lists the result of regression analysis. The F value of model Fisher's exact test and p value of the model t-test were 21.495 and 0.000, respectively, indicting that the regression analysis was reliable and statistically significant, and the correlation relationship between Y and each factor was extremely significant. Moreover, the coefficient of Fi2 was less than 0, indicating that there were inverted U-shape relationships between the TP production and DS amount or each operational condition. In addition, the p values of F12, F1, F22, F2, F42, F4, F52, F5, F62 and F6 were less than 0.01, suggesting that the effects of DS, inoculation volume, incubation temperature, corn hull and water-material ratio on the TP production were extremely significant. The effect of incubation time was not significant within the test range. According to the coefficient of each item of the Y (the TP production) (Table 4), the Y could be expressed by the equation as follows (2):
 |
| Co-efficient | t | p | |
|---|---|---|---|
| constant | −159.559 | −8.478 | 0.000 |
| F12 | −20.668 | −3.832 | 0.000 |
| F1 | 16.669 | 3.369 | 0.001 |
| F22 | −0.037 | −3.574 | 0.001 |
| F2 | 1.420 | 4.645 | 0.000 |
| F32 | −0.098 | −0.400 | 0.680 |
| F3 | 2.529 | 0.738 | 0.453 |
| F42 | −0.083 | −10.376 | 0.000 |
| F4 | 4.415 | 10.904 | 0.000 |
| F52 | −0.143 | −6.506 | 0.000 |
| F5 | 4.364 | 6.538 | 0.000 |
| F62 | −91.471 | −8.329 | 0.000 |
| F6 | 155.432 | 8.764 | 0.000 |
Model: F = 21.531; p = 0.000
The following equation was obtained by taking the derivation of Y to each Fi
 |
 |
 |
 |
 |
 |
If Y'=0, F1=0.403, F2=19.2, F3=12.9, F4=26.6, F5=15.2, F6=0.85. In other words, the optimum culture condition included DS of 0.403 g, inoculation volume of 19.2 mL, incubation time of 12.9 d, incubation temperature of 26.6 °C, and corn hull of 15.2 g at a water-material ratio of 0.85. Under the optimum condition and medium, the theoretical maximum TP production could reach 115.6 mg/g dry substrate.
Effect of inoculation volume on the TP production The optimum inoculation volume is an extremely significant factor for TP production. Too small inoculation volume can make the biomass rapidly cover the whole substrate, leading to contamination by undesirable organisms. Conversely, too large inoculation volume can generate too much biomass, leading to the increased consumption of the substrate and resulting in a poor TP production (Mudgett and Paradis, 1985; Mudgett, 1986). According to results of regression analysis, we could deduce that optimum inoculation volume was 19.2 mL. When the inoculation volume was increased from 5 mL to 19.2 mL, the TP production was gradually increased. When the inoculation volume was increased over 19.2 mL, the TP production would be gradually decreased. Similar result has been found in the process of Monascus pigments (Lee, et al., 2002) and LSF of T. melanosporum (Zhang, et al., 2019).
Effect of incubation temperature on the TP production As another extremely significant factor, incubation temperature can affect TP production in the process of SSF. Because of the mesophilic nature of T. melanosporum, maximum TP production was obtained at 26.6 °C according to non-linear analysis between DS and operational conditions. A reduction in TP production was detected when the incubation temperature was higher or lower than 26.6 °C. Therefore, the incubation temperature was important for the TP production in the process of SSF. Our results were consistent with previous studies (Liu and Tang, 2010; Zhang, et al., 2019) that the optimum temperature for T. melanosporum ranges from 25 °C to 28 °C. Generally, the optimum temperature of SSF is 1.5–2.5 °C less than that of LSF. This could be attributed to the poor heat diffusion in the process of SSF. The poor heat diffusion results in the accumulation of the bio-heat in the medium and further causes the reduction in metabolic activity of mycelia and TP synthesis.
Effect of air-permeability on the TP production Air-permeability plays a crucial role in TP production. Extremely low air-permeability inhibits the fungal metabolism, especially for aerobic metabolism. In contrast, extremely high air-permeability promotes the water evaporation and reduces the synthesis of TP. In the present study, the air-permeability was adjusted by corn hull and water-material ratio. The higher amount of corn hull added, the higher the air-permeability. The regression analysis on the synergic effect between DS and operational conditions on TP production showed that the optimum amount of corn hull was 15.2 g. When the amount of corn hull added was increased from 9 g to 15.2 g, the TP production was rapidly increased to maximum. When the amount of corn hull added was further increased, the TP production was decreased.
Effect of water-material ratio on the TP production Regression analysis of correlation between DS and operational conditions showed that the maximum TP production was achieved when the water-material ratio was 0.85. The water in the medium may exert three impacts on the system. Firstly, it will tend to favor the hydrolysis reaction. Secondly, such ratio influences the enzyme's microenvironment, which in turn impacts the catalytic activity of intra-cellular enzymes and susceptibility to denaturation (Liu, et al., 2010; Liu, et al., 2011; Salum, et al., 2010). Thirdly, water adjusts the air-permeability of medium, which affects the growth of the strain. The radical difference between the submerged fermentation and SSF is the mass and heat exchange way. In the process of SSF, most microorganisms directly contact with the gaseous oxygen, and its low water-material ratio is favorable compared with the submerged fermentation. The mass and heat exchanges in the SSF process, namely the overall reactions, directly take place between gas phase and solid phase in the process of SSF. The mass exchange includes the transport of oxygen and water into inner of the microbial biomass. The heat exchange namely is the generation of metabolic heat due to respiration. The heat and carbon dioxide are transported from the interior substrate into the gas phase. The whole mass exchange process directly take place between gas phase and solid phase in the process of SSF. As one of the most important factors, the water-material ratio of substrate impacts the mass exchange and heat release. Extremely high water-material ratio decreases the air-permeability of medium, which does not favor the transport of oxygen and change of mass. In contrast, extremely low water-material ratio leads to denaturation of intracellular enzymes and reduces the catalytic activity of these enzymes.
The effect of incubation time on the activity of fermented solid Regression analysis of correlation between DS and operational conditions indicated that the maximum TP production was achieved after 12.9 d of incubation. This might be attributed to the consumption of the nutrients or denaturation of the enzymes caused by the interaction with other components in the medium or change in the medium pH (Fernandes, et al., 2007). This incubation time was longer than the fermentation time of LSF (Zhang, et al., 2019). The incubation time is also a crucial factor of SSF. However, the regression analysis showed that effect of incubation time was not significant. This could be attributed to that our designed experimental incubation time was far less than the theoretical value.
To verify the optimized results, the optimum medium was composed of 60 g oat, 15.2 g corn hull, 1.8 g fructose, 1.2 g sucrose, 0.48 g peptone, 0.72 g yeast extract, 0.06 g MgSO4·7H2O, 0.12 g MnSO4, 0.09 g KH2PO4·H2O, 0.01 g VB2 and 0.4 g DS at a water-material ratio of 0.85. The medium was sterilized at 121 °C for 1 h. After 19.2 mL liquid seed was added, the fermentation was conducted at 26.6 °C for 12.9 d. The TP production was 106.7 ± 2.4 mg/g dry substrate, which was close to the theoretical maximum production of TP and increased 5.6 times compared with the non-optimized value (18.79 mg/g dry substrate).
In this paper, the optimal medium and culturing condition has been obtained by orthogonal matrix and nonlinear regression analysis. Under the optimal conditions, the maximum TP production was achieved, which was 106.7 ± 2.4 mg/g dry substrate and close to theoretical maximum production of TP. The method for the fermented oat production rich in TP was developed by SSF with T. melanosporum. In addition to oat, many traditional foods with starch could be used as main components, including rice, buckwheat and broomcorn. To enhance the bioactive ingredients and biomedical functions of these traditional foods, SSF of T. melanosporum with these starchy foods could be one ideal choice. Moreover, natural food fermented by edible and medical mushrooms under SSF could be applied in the production of nutritious food. Moreover, nonlinear regression analysis was a better method, which could make up the deficiency of our experimental design.
Acknowledgments This research was funded by the Natural Science Foundation of Jiangsu Province, grant number BK20160493; the China Postdoctoral Science Foundation, grant number 2015M571691; the Senior Talent Scientific Research Initial Funding Project of Jiangsu University, grant number 15JDG17.