2020 Volume 26 Issue 4 Pages 535-543
2020 Volume 26 Issue 4 Pages 535-543
Salivation is indispensable for the smooth intake of food and maintenance of oral health. In this study, we investigated how we recognize foods in our mouth, with a focus on the salivary reflex response. Mastication of deacylated gellan gum (DGG) and native gellan gum (NGG) gels induced relatively higher salivation than that induced by the mastication of agar gel, which showed high syneresis. Furthermore, salivation correlated positively with masseter muscle activity for the mastication of agar and DGG gels showing relatively high elasticity and low plasticity. The increase in food volume consumed at one time increased salivation only within a relatively small range; however, the increase in surface area of gels did not affect salivation for the mastication of agar gels. These results enhance our basic understanding of the oral processing of food and provide guidelines for designing a salivation-promoting meal.
Salivation is indispensable for the smooth intake of food and maintenance of oral health. Salivation induced by food-derived oral stimulation to facilitate mastication and swallowing is known as stimulated salivation (Pedersen et al., 2018). A relatively small amount of salivation that is autonomously secreted to condition the pH, humidity, and microbial condition of the oral cavity throughout the day is known as resting salivation (Proctor, 2016; Pedersen et al., 2018; Feron, 2019). Salivation is closely related to healthy and palatable eating. Poor salivation interferes with swallowing of food and leads to dry mouth (xerostomia) and imbalanced oral microbiota. Thus, salivation is a critical factor affecting the quality of life of people.
To improve our eating experience, it is essential to understand the relationship between salivation and mastication because it affects the recognition of foods in the mouth. The effects of mastication, including the number of chews and duration of chewing, on salivation have been studied using non-digestible paraffin and chewing gum in clinical settings (Jensen et al., 1998; Rayment et al., 2001; Gavião et al., 2004; Dawes and Kubieniec, 2004). These studies showed that frequent chewing (i.e., a high number of times a food is chewed per minute) induces more salivation, especially in the first minute of mastication (Jensen et al., 1998; Rayment et al., 2001; Gavião et al., 2004). After reaching the highest salivary flow rate, prolonged chewing results in constant salivation, which is less than maximum salivation but much more than resting salivation (Rayment et al., 2001; Dawes and Kubieniec, 2004). Additionally, Kerr (1961) showed a positive correlation between chewing mass and salivation using wax. However, the process of masticating natural foods differs from that of masticating non-digestible foods, without swallowing.
Salivation induced by mastication is in the following order: natural foods > chewing gum > paraffin (Mackie and Pangborn, 1990; Gavião et al., 2004; Jensen et al., 1998). The number of times a food needs to be chewed and the duration of chewing until swallowing vary among natural foods, depending on constituent factors such as water and fat contents (Gavião et al., 2004). The volume of natural foods also affects the number of chews and the duration of chewing until swallowing, although its effect on salivation is controversial (Mackie and Pangborn, 1990; Gavião et al., 2004). Stimuli from natural foods are derived from a diverse combination of tastes, flavors, textures, and physical characteristics. Salivation during the oral processing of natural foods is determined by the sum effect of each constituent factor. Therefore, the function of each constituent factor in salivation should be analyzed separately to gain basic knowledge of the oral processing of natural foods.
In this study, we aimed to identify the physical factors of a bite of food that stimulate salivation. We used water and 3 types of hydrocolloid gels with different physical characteristics. These gels can be swallowed like natural foods, and the mechanical properties of gels are easy to control compared with those of natural foods (Ishihara et al., 2013, 2014; Koç et al., 2014; Kohyama et al., 2015). We measured masseter muscle activity and salivation simultaneously in individuals to quantify the effects of fracture force, apparent elastic modulus, bite size (volume consumed at one time), and surface area of sample gels on oral processing.
Subjects Twenty-six healthy, nonsmoking volunteers aged 19–48 years (average age = 25.4 years) participated in this study. The volunteers tested 4–9 samples per session in the laboratory. Two sessions were conducted per day; the morning session started at 10:00, and the afternoon session started at 13:00. This research was conducted in accordance with the principles of the Declaration of Helsinki adopted by the World Medical Association and approved by the ethical review committee of the Food Research Institute, National Agriculture and Food Research Organization (Approval number 29NFRI-0001).
Hydrocolloid gels Three types of gels kindly provided by San-Ei Gen, F.F.I., Inc. (Osaka, Japan) were prepared: 0.5 % w/v agar, 0.5 % w/v deacylated gellan gum (DGG with 0.2 % w/v calcium lactate, and 2.0 % w/v native gellan gum (NGG) with 0.1 % w/v calcium lactate. Each gelling agent was dissolved in deionized water at 90 °C for 10 min. Calcium lactate was separately dissolved in deionized water at 90 °C and then added to the DGG and NGG solutions. The desired volume of each solution was obtained by adding deionized water. Each solution was then poured into glass ring molds (20-mm diameter; 10-mm height; 3.14-mL volume) (Ishihara et al., 2013, 2014; Kohyama et al., 2014, 2016b, 2017). The samples in molds were heated at 85 °C for 30 min and then refrigerated at 4 °C for at least 1 h. Gels were allowed to stand at 23 °C for 1–3 h before using for experiments.
Procedures
Experiment 1: Measurement of salivation during oral processing of water and gels All samples were tested at 23–27 °C (room temperature). The test interval was 20–21 min, and each session lasted for 65–125 min. Two experiments were conducted: [1] fourteen subjects tested 4–7 samples, which included 1 and 3 pieces each of agar, DGG, and NGG gels, and 3.14 and 9.42 mL of water, equivalent to 1 and 3 pieces of gels, respectively, in the morning session and/or afternoon session; [2] six subjects tested 5 samples, which included 1, 2, 3, and 4 pieces of agar gel and 3.14 mL of water, equivalent to 1 piece of gel, in the morning session and/or afternoon session.
Experiment 2: Measurement of masseter electromyographic (EMG) activity and salivation during oral processing of water and gels Ten subjects tested 9 samples in the afternoon session, and EMG activity was recorded at this time. All samples were tested at 23 °C (room temperature). The test interval was 20–21 min, and the duration of the session was 165 min. Samples used were 1 and 3 pieces each of agar, DGG, and NGG gels; 3.14 mL of water; and crushed 1 piece of agar gel (crushed by hand and squeezed out of a tube with a 5-mm diameter opening). One piece of agar gel was examined twice (one control and one experimental). Data collected from one of the subjects were excluded from the analysis because the amount of salivation showed negative values, indicating that the subject swallowed a part of the sample.
Salivation measurement The subjects washed their mouths with activated carbon-filtered water (Water purifier TK-AS44, Panasonic Corporation, Osaka, Japan) approximately 2 min before each test. Subjects were instructed to take sample gels placed on a plastic spoon or equivalent volumes of water in a cup at one time, masticate for 30 s, which was sufficiently long considering the duration of mastication for 3 g of hydrocolloid gels (Kohyama et al., 2014; 2016a), and expectorate all of the oral contents, including the saliva secreted during mastication, into the same cup. Immediately afterwards, saliva was collected into the same cup for 60 s.
The difference in the weight of the cup between before and after a test was determined as the amount of saliva (g) secreted in 90 s, and the rate of salivation (g/min) was calculated. The normalized salivation (a.u.) in Experiment 1[2] was defined as the ratio of the rate of salivation (g/min) for each sample to that for 3.14 mL of water (g/min) in each subject. The normalized salivation (a.u.) in Experiment 2 was defined as the ratio of the rate of salivation (g/min) for each sample to that for the control experiment using 1 piece of agar (g/min) in each subject for consistency with the EMG analysis described in the next section.
EMG measurement EMG activity was recorded from a masseter muscle on the side opposite to the dominant arm of the subject using bipolar surface electrodes filled with electrode gel (COVEDIEN H124, Medtronic Japan Co., Ltd., Tokyo, Japan). The electrodes were placed 2.5 cm apart from each other on the masseter muscle along the muscle fibers. The reference electrode was placed on the same side of the mastoid process (Ferella et al., 2008; Iwasaki et al., 2016). Electrodes were connected to a portable biosignal logging device, and data were recorded at 1 000 Hz (Biosignal plux, PLUX Inc., Lisbon, Portugal).
The cumulative masseter EMG activity (mV·s) was calculated as an integral of the raw EMG amplitude. The normalized EMG activity (a.u.) was defined as the ratio of the raw cumulative EMG activity (mV·s) at each time point to that for 30 s of the control agar gel (mV·s) in each subject because water processing, which yielded almost no EMG activity, was inadequate to normalize EMG activity.
Syneresis measurements One piece of intact gel was weighed immediately after removed from the mold (t = 0 h) and allowed to stand in a vessel with food wrap at 23 °C. The liquid released from each intact gel was weighed at each time point. The syneresis (%) was calculated as the ratio of the total weight of released liquid to the initial weight of gel × 100.
Mechanical measurements The mechanical properties of gels were analyzed using a universal testing machine equipped with a 500-N load cell (Instron 5542, Canton, MA, USA). Gels were compressed using a flat stainless steel probe (2 840 mm2) at a constant velocity of 10 mm/s at 23 °C. Gel compression was stopped when the height of the gel reached 20 % of the initial height. The first peak in the force-deformation curve was defined as the fracture point. If the fracture point was observed, the values of fracture force, fracture deformation ratio (ratio of fracture deformation to the initial gel height), and fracture work (area under the curve until the first peak) were determined. Apparent elastic modulus (Young's modulus) was calculated at a point of 0.5 mm compression, assuming that the contact area was the same as the initial condition (314 mm2).
Statistical analysis Differences between 2 groups were assessed using a Student's t-test, and differences among ≥ 3 groups were assessed using one-way analysis of variance (ANOVA), followed by post hoc pairwise comparisons with Holm's correction. Pearson's product-moment correlation coefficients were calculated to determine the relationships between salivation and masseter EMG activity. Differences were considered significant at p < 0.05.
Salivation during oral processing of water and gels Salivation during the mastication of agar, DGG, and NGG gels was significantly more than that during the oral processing of water (Experiment 1[1]; Fig. 1A). Mastication of 1 piece (3.14 mL) of agar gel stimulated a moderate increase in salivation compared with that during the oral processing of an equivalent volume of water, whereas the mastication of 1 piece of DGG and NGG gels stimulated greater salivation than the oral processing of water (Fig. 1A, left panel). Similar results were obtained using 3 pieces (9.42 mL) of all the gels. Additionally, salivation during the mastication of 1 piece of NGG gel was significantly greater than that during the mastication of 1 piece of agar (Fig. 1A, right panel).

Comparison of salivation during the mastication of different volumes of gels or water (Experiment 1[1]). A. Salivation for the mastication of 3 types of gels and water. Left: mastication of 1 piece of agar (n = 11), DGG (n = 14), and NGG (n = 14) gels and an equivalent volume of water (3.14 mL; n = 14). Right: mastication of 3 pieces of agar (n = 11), DGG (n = 14), and NGG (n = 14) gels and an equivalent volume of water (9.42 mL; n = 13). Data are presented as mean ± standard error of mean (SEM). B. Increase ratios of salivation for mastication of 1 piece to 3 pieces of gels. Significant differences were detected using one-way ANOVA, followed by post hoc pairwise comparisons with Holm correction. ***, p < 0.005; *, p < 0.05; n.s., not significant.
Mastication of 3 pieces of gels tended to promote more salivation than that with mastication of 1 piece. The increase in salivation during the mastication of 3 pieces of gels compared with that of 1 piece was similar among the 3 types of gels and water (1.17–1.22-fold), with no significant differences (Fig. 1B; p > 0.05).
To examine the effect of the volume of agar gel on salivation in detail, the amount of salivation during the mastication of 1, 2, 3, and 4 pieces of agar gel was quantified Experiment (1[2]). To calculate the induction ratio of salivation for mastication of agar gels compared to that for oral processing of water, the amount of salivation was normalized to the salivation induced by oral processing of 3.14 mL of water. The salivation induced by mastication of 1 piece of agar gel was more than twice that induced by the processing of the same volume of water (Fig. 2). The average of individually normalized salivation increased monotonically with the increase in agar gel volume (Fig. 2). Significant differences in salivation were detected between 1 and ≥ 2 pieces of agar gel. Once the volume of agar gel exceeded 2 pieces, no significant increase in salivation was detected.

Effect of the volume of gel consumed at one time on salivation for mastication (Experiment 1[2]). The amount of salivation for 1–4 pieces of agar gels were normalized to that for 3.14 mL of water, which was equivalent to 1 piece of gel. Data are presented as mean ± SEM. Significant differences were detected using one-way ANOVA, followed by post hoc pairwise comparisons with Holm correction. **, p < 0.01; *, p < 0.05.
Physical characteristics of gels Differential salivation for mastication of different types of gels was presumably caused by differences in the physical characteristics of these gels. The agar gel kept at room temperature released a large amount of liquid compared to the other gels. The ratio of syneresis of agar gel was 5.0-fold higher than that of DGG gel at 6 h (Fig. 3). The early syneresis from DGG gels was similar to that from agar gels at 1 h. However, DGG gels did not show additional syneresis, whereas agar gels continued to release liquid until 6 h later. NGG gels showed no syneresis at the 6 h measurement (Fig. 3).
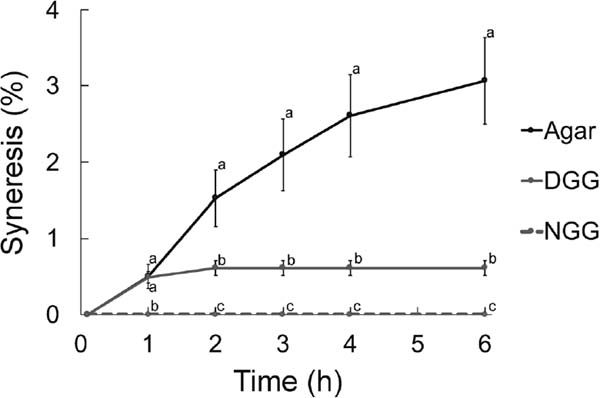
Analysis of syneresis from 1 piece of intact gels. The syneresis (%) of agar (black solid line, n = 5), DGG (gray solid line, n = 5), and NGG (gray dashed line, n = 5) gels are shown. Data are presented as mean ± SEM. Different alphabetical letters indicate significant differences, as determined by one-way ANOVA (p < 0.005), followed by post hoc pairwise comparisons with Holm correction (p < 0.05).
The force-deformation ratio curves also significantly differed among the 3 types of gels (Fig. 4). Results of mechanical analyses revealed that the fracture force of DGG gels was the highest, 4.5-fold higher than that of agar gels (Table 1). The fracture deformation ratios of DGG and agar gels were similar, whereas the fracture work of DGG gels was 6.4-fold higher than that of agar (Table 1). The fracture point was observed in agar and DGG gels; conversely, NGG gels did not clearly fracture under the experimental conditions used in this study. NGG gels extended to a thin and flat layer under a relatively low compression force, indicating that NGG gels exhibit higher plasticity than agar and DGG gels. The NGG gel was soft, with low apparent elastic modulus (Table 1, Fig. 4), and did not exhibit force peak even under a load of 1000 N (data not shown). The apparent elastic modulus of DGG gels was significantly higher than that of the other gels; it was 7.1- and 19.2-fold higher than that of agar and NGG gels, respectively (Table 1).

Mechanical measurement to analyze the physical characteristics of different gel types. Mechanical forces needed to compress agar (black solid line, n = 7), DGG (gray solid line, n = 6), and NGG (gray dashed line, n = 6) gels are shown. The peak compression force was determined as a fracture point, which was observed only in agar and DGG gels. Data are presented as mean ± SEM.
| Agar (n = 7) |
DGG (n = 6) |
NGG (n = 6) |
|
|---|---|---|---|
| Fracture force (N) | 9.62 ± 0.63 a | 43.67 ± 2.18 b | Not detected |
| Fracture deformation ratio (m/m) | 0.42 ± 0.01 a | 0.44 ± 0.01 a | Not detected |
| Fracture work (N·mm) | 14.79 ± 1.43 a | 94.08 ± 4.41 b | Not detected |
| Apparent elastic modulus (kPa) | 4.77 ± 0.78 b | 33.78 ± 9.32 c | 1.76 ± 0.57 a |
Data represent mean ± Standard error of mean (SEM).
Different alphabetical letters within a row indicate significant differences, as determined by one-way (ANOVA) followed by post hoc pairwise comparisons with Holm correction (p< 0.05).
Masseter EMG activity and salivation To understand the relationship between the physical characteristics of gels and salivation, we measured masseter EMG activity to quantify the intensity of mastication using agar, DGG, and NGG gels (Table 2, Fig. 5). Salivation was also measured simultaneously with masseter EMG activity (Experiment 2). No significant effects of gel volume (1 piece or 3 pieces) were detected in the EMG activities for 30-s mastication of agar, DGG, and NGG gels (Fig. 5A). Three pieces of NGG gel produced significantly higher EMG activity than the other gels. The EMG activity for 1 piece of NGG gel did not differ significantly from that for 1 and 3 pieces of DGG gel, while EMG activity for 1 piece of DGG gel was similar to that for 1 and 3 pieces of agar gel. The cumulative EMG activity for 1 piece of NGG gel was slightly smaller than that for 3 pieces of DGG gel in the early stage (< 7 s), although it increased rapidly in the following period, similar to that for 3 pieces of NGG gel (Fig. 5B). The cumulative EMG activity for 1 piece of DGG gel was similar to that for 1 and 3 pieces of agar gel in the early stage, and continued to increase constantly in parallel with that for 3 pieces of DGG gel in the latter half (Fig. 5B). Importantly, EMG activity and salivation showed a significant positive correlation with respect to agar and DGG gels, but not NGG gels (Fig. 5C).
| Water | Crushed agar | Agar | DGG | NGG | |
|---|---|---|---|---|---|
| 1 piece (3.14 mL) | |||||
| Raw EMG activity in 30 s (mV·s) | 133 ± 20 | 230 ± 38 | 257 ± 25 | 333 ± 43 | 597 ± 106 |
| Normalized EMG activity in 30 s (a.u.)† | 0.60 ±0.11 | 0.91 ± 0.09 | 1.01 ± 0.06 | 1.34 ± 0.09 | 2.34 ± 0.31 |
| 3 Dieces (9.42 mL) | |||||
| Raw EMG activity in 30 s (mV·s) | 305 ± 47 | 406 ± 52 | 804 ± 124 | ||
| Normalized EMG activity in 30 s (a.u.)† | 1.20 ± 0.09 | 1.63 ± 0.13 | 3.23 ± 0.43 | ||
Data represent mean ± SEM of 9 subjects.

Correlation between the masseter electromyographic (EMG) activity and salivation for mastication of flavorless gels (Experiment 2). The EMG activity and salivation were measured simultaneously for 30 s. Normalized EMG activity (a.u.) was defined as the ratio of the raw cumulative EMG activity (mV·s) in each time point to that for 30 s of control experiment (mV·s) using 1 piece of agar gel in each subject. Normalized salivation (a.u.) was defined as the ratio of the rate of salivation (g/min) for each sample to that for the control experiment (g/min) in each subject. A. Comparison of the EMG activity during 30-s mastication of 1 and 3 pieces of agar, DGG, and NGG gels (n = 9 for each). Data are presented as mean ± SEM. Different alphabetical letters attached indicate significant differences, as determined by one-way ANOVA (p < 0.005) followed by post hoc pairwise comparisons with Holm correction (p < 0.05). B. The cumulative time course of EMG activity during the mastication of 1 piece of agar (gray dashed line), 3 pieces of agar (black dashed line), 1 piece of DGG (gray solid line), 3 pieces of DGG (black solid line), 1 piece of NGG (gray dotted line), and 3 pieces of NGG gels (black dotted line; n = 9 for each). Data are presented as mean ± SEM. C. Correlation between the EMG activity and salivation during 30-s gel mastication. Each plot shows the results of 1- and 3-piece mastication test (n = 18 for each gel). r, Pearson product-moment correlation coefficient; **, p < 0.01; *, p < 0.05.
Effect of food surface area on salivation In the case of agar gels, both volume and physical characteristics affected salivation to some extent, and it seemed to occur in the boundary of the physical property of water and solid gel. To examine the difference between the oral recognition of water and that of gels, 1 piece of agar gel, an equivalent volume of crushed agar gel, and an equivalent volume of water (3.14 mL) were compared (Experiment 2; Fig. 6A). Salivation was significantly greater during the mastication of crushed and intact pieces of agar gel than during oral processing of water (Fig. 6A). EMG activities were also higher during the mastication of crushed and intact pieces of agar gel compared to oral processing of water, although the difference was significant only between 1 piece of agar and water (Fig. 6A; Table 2). Salivation and EMG activity during the mastication of 1 piece of agar gel were only slightly higher than those during the mastication of crushed agar gel (Fig. 6A). No significant correlation was detected between EMG activity and salivation in the processing of 3.14 mL of crushed agar or water (Fig. 6B).

Comparison of the mastication of crushed and intact agar gel and oral processing of water (Experiment 2). Volumes of water and crushed agar gel were equivalent to 1 piece of intact agar gel. The EMG activity and salivation were measured simultaneously for 30 s. Normalized salivation (a.u.) and normalized EMG activity (a.u.) for each sample were defined as the same as shown in Fig. 5. A. Salivation (left) and EMG activity (right) during 30-s oral processing of water and mastication of the crushed and intact single piece of agar gel (n = 9 for each sample). Data are presented as mean ± SEM. Data on 1 piece of agar are the same as shown in Fig. 5A. Significant differences were determined using one-way ANOVA (p < 0.005), followed by post hoc pairwise comparisons with Holm correction. ***, p < 0.005; *, p < 0.05. B. Correlation between the EMG activity and salivation during the 30-s oral processing of water and crushed agar gel. r, Pearson product-moment correlation coefficient. No significant correlations were detected between salivation and EMG activity for oral processing of water and mastication of crushed agar gel.
In this study, we showed that salivation for mastication depends on the physical characteristics of gels and correlates with individual masseter EMG activity, as for agar and DGG gels. The increase in the volume of agar gel consumed at one time induced salivation when the volume was sufficiently small (< 6 mL), suggesting that the maximum amount of salivation is determined by the volume and physical characteristics of food.
Measurement of masseter EMG activity enables an objective quantification of the mastication effort (Brown et al., 1994, Gonzalez et al., 2001; Bourne, 2002; Kohyama et al., 2003). Additionally, relative EMG activities for different samples have been reported to be similar among subjects, despite the large inter-subject variation in EMG activity (Kemsley et al., 2003; Brown et al., 2004; Kohyama et al., 2016c). Simultaneous measurement of EMG activity and salivation in this study revealed a significant correlation between individual EMG activity and salivation for mastication of both agar and DGG gels. Our mechanical measurement showed that the fracture force and fracture work of DGG gels were significantly larger than those of agar gels, although the fracture deformation ratio was similar between DGG and agar gels. These observations are consistent with the findings of previous studies showing that the masticatory muscle activity was higher for foods with high fracture force, work, and deformation ratio (Funami et al., 2014; Kohyama et al., 2016b). The EMG activity for mastication of 1 piece of DGG gel was significantly higher than that for agar gels, suggesting that DGG gels, characterized by a large fracture force and fracture work, require more mastication effort than agar gels and result in greater salivation compared with agar gels. Mastication of gels with high EMG activity induces more salivation. This finding may explain why salivation for the mastication of tough and dry beef was higher than that for the mastication of tender and juicy beef (Mioche et al., 2004).
However, we observed no clear correlation between salivation and EMG activity for the mastication of NGG gels, despite the relatively high EMG activity of NGG gels compared to agar and DGG gels. Oral processing of NGG gels, characterized by low elasticity and high plasticity, appeared to differ from that of agar and DGG gels, which showed high elasticity and low plasticity. Our mechanical measurements showed that NGG gels were hardly fractured into several pieces by compression, suggesting that the compression work does not decrease in a stepwise manner through chewing cycles. A possible explanation for the high EMG activity for the mastication of NGG gels is the ongoing large compression work for every chewing cycle. It is important to note that after the test session, some subjects reported that they could not masticate sufficiently in 30 s, whereas mastication for 30 s was sufficient for other gels. This difficulty in masticating NGG gels possibly caused individual variation in the EMG activity. Although there was no clear correlation, the mastication of NGG gels induced, on average, significantly more salivation than that of agar gels. This result also supports the general correlation between salivation and mastication effort.
The amount of salivation for the mastication of agar gels was about two-thirds of that for DGG and NGG gels. The ratio of syneresis of agar gels was higher than that of DGG and NGG gels, suggesting that the liquid released from agar gels may partly complement the salivation required for mastication.
The increasing ratios of salivation according to the increase in volume from 3 to 9 mL were similar between water and gels. However, the differences in the amount of salivation for mastication of 3 and 9 mL (1 and 3 pieces) were not significant in any of these cases, possibly because of large individual variations. Hence, we examined the effect of the increase in volume on individually normalized salivation using agar gels as a representative and observed that the increase in the volume of agar gels induced salivation only with gels < 6 mL, and little effect was observed when the volume was > 6 mL. This suggested that the volume of gel consumed at one time affects salivation only when the volume is sufficiently small; a volume over a certain threshold does not stimulate additional salivation. A previous study showed that the volume of food consumed at one time affects the number of chews and duration of mastication but not the masseter muscle activity per chew (Kohyama et al., 2014, 2016a). Unfortunately, the number of chews and the duration of mastication could not be evaluated in this study because we set the duration of mastication to be 30 s. In fact, EMG activities in 30 s did not differ significantly between mastication of 1 piece and 3 pieces of each gel type, suggesting that the increase in salivation for mastication of ≥ 2 pieces compared with 1 piece was hardly caused by the difference in EMG activity. The amount of salivation is directly correlated with the formation of a food bolus for swallowing (Hutchings and Lillford, 1988; Peyron et al., 2017). Thus, the finding that the volume of food affects salivation only within a small range indicates that a small amount of salivation is sufficient for swallowing.
The surface area of gels is another possible factor affecting salivation. We compared salivation during the mastication of 1 piece of agar gel with that during the mastication of an equivalent volume of crushed agar gel, which has a larger surface area than that of the intact agar gel. The result showed no significant differences in both salivation and EMG activity, indicating that the change in physical characteristics between crushed and intact agar gels has almost no effect on EMG activity, and thus, the increase in surface area of agar gels does not stimulate salivation. The effect of temperature, a factor affecting salivation, could be excluded because the temperature of all samples was similar.
Mastication of gels including crushed agar induced significantly more salivation than an equivalent volume of water. Oral recognition of any small solid fragments stimulated more salivation than water. These results are consistent with those reported by Mackie and Pangborn (1990), who showed that oral mechanical stimulation derived from solid food, but not liquid food, is a factor in inducing salivation. The relationship between salivation and physical characteristics of food (without mastication) and swallowing was not examined in our study because we focused primarily on salivation alone during the mastication of different gels within the same time frame (30 s). Further investigation is needed to clarify the overall relationship among salivation, physical characteristics, oral recognition, mastication, and swallowing.
The results of this study involving the use of 3 types of gels, which are not “natural food” but rather “artificial food,” enhanced our basic understanding of the oral processing of food. This would help in the design of natural foods that induce salivation to promote smooth food intake. The abilities of both stimulated and resting salivation decline with aging (Percival et al., 1994; Yeh et al., 1998; Affoo et al., 2015). A meal designed to promote natural salivation, as an alternative to symptomatic treatment using artificial materials, is ideal to aid in low salivation upon eating as well as reduce stress and promote palatable eating (Jensen et al., 1998; Gil-Montoya et al., 2016; Vandenberghe-Descamps et al., 2017). We propose that high-elasticity and low-plasticity foods such as DGG gels, which can evoke masseter muscle activity, are useful as a salivation-promoting meal. Importantly, eating a moderate volume of food several times is better for promoting salivation than eating a large volume of food at once.
Furthermore, agar and DGG gels with a certain taste and flavor would serve as useful model foods to understand the relationship between salivation and oral recognition upon eating natural foods, as mastication of tasteless and flavorless gels stimulates salivation according to the masseter muscle activity. A future challenge is to establish a functional food design, with the integration of tastes and flavors.
Acknowledgments This work was supported in part by the Mishima Kaiun Memorial Foundation and by JSPS KAKENHI Grant Number JP19H01618.