2020 Volume 26 Issue 5 Pages 633-642
2020 Volume 26 Issue 5 Pages 633-642
A simple, efficient, rapid, low-cost and reliable method for the determination of herbicide residues by high performance liquid chromatography- photodiode array detection (HPLC-DAD) was established. The recoveries of quizalofop-p-ethyl and its metabolite quizalofop-p-acid were between 88.7 and 116.2%, and the relative standard deviations (RSDs) were between 0.82 and 4.39%. The limits of detection (LODs) were 0.005–0.008 mg/kg for quizalofop-p-ethyl and 0.003–0.01 mg/kg for quizalofop-p-acid. The limits of quantification (LOQs) were 0.015–0.02 mg/kg for quizalofop-p-ethyl and 0.01–0.03 mg/kg for quizalofop-p-acid. The method was reliable and stable. The residual quizalofop-p-ethyl and its metabolite quizalofop-p-acid in adzuki bean seeds, plants and soils from Zhangjiajie (Hunan) and Anda (Heilongjiang) were determined. Under different climatic conditions in central and northern China, the residual dynamics of quizalofop-p-ethyl in soils, plants and seeds of adzuki bean were studied. The results showed that the half-life of quizalofop-p-ethyl in adzuki bean plants and soils was between 3.4 and 6.7 d. The residues of quizalofop-p-ethyl and its metabolite quizalofop-p-acid in adzuki bean seed, plant and soil were determined to be lower than the maximum residue limit of 0.05 mg/kg.
The seeds of adzuki bean are rich in protein, and have the content of 8 essential amino acids 2–3 times higher than cereals (Osorio-Diaz et al., 2003; Siddiq et al., 2010). They are also rich in dietary fiber, starch, minerals and vitamins, and thus have high nutritional value (Han et al., 2011; Jhan et al., 2015). With the improvement of people's living standards and the deeper understanding of adzuki beans, people demand more and more adzuki bean.
To increase the yield of adzuki beans, farmers inevitably use herbicides to remove weeds from their fields. Quizalofop-p-ethyl is the most widely used selective herbicide in soybean to control annual and perennial grass weeds (Yadav et al., 2017). Both quizalofop-p-ethyl and its metabolite quizalofop-p-acid can inhibit the synthesis of fatty acids in the target weeds, leading to growth inhibition and deterioration (Mantzos et al., 2016). Both compounds have been shown to be genotoxic (Elefsiniotis et al., 2007; Mustafa and Arikan, 2008; Doganlar 2012). When applied to adzuki beans, residues of quizalofop-p-ethyl and its metabolite pose potential risks to the environment and the public health. According to the maximum residue level (MRL) database prepared by the United States Department of Agriculture (i), the maximum permissible residue level of quizalofop-p-ethyl used in soybean seed production in the United States is 0.05 mg/kg. The maximum residue limit established by the Chinese Ministry of Agriculture is 0.1 mg/kg.
Currently, several analytical methods have been reported for the determination of quizalofop-p-ethyl in food, water and soil substrates, such as ultra high performance liquid chromatography (UHPLC) combined with Orbitrap mass spectrometry (MS) (López-Ruiz et al., 2017), high performance liquid chromatography (HPLC) and diode-array detection (DAD) (Saha et al., 2016; Ma et al., 2016), liquid chromatography (LC)-(DAD) (Lubomirsky et al., 2016), gas chromatography (GC) mass spectrometry (Mantzos et al., 2016), and HPLC-MS (Guan and Zhang, 2013). However, the simultaneous detection of quizalofop-p-ethyl and its metabolite in adzuki bean has not been reported in the literature. Although MS detectors are very sensitive, they are also very expensive (Lubomirsky et al., 2016). Considering the economics and the capacity of ordinary laboratories, HPLC-DAD was used to detect quizalofop-p-ethyl and its metabolite.
The sample preparation methods used for extraction of quizalofop-p-ethyl from various matrices mainly include QuEChERS (quick, easy, inexpensive, effective, rugged, and safe) method (Li et al., 2013; Wang et al., 2018; He et al., 2018), solid phase extraction (SPE) (Hu and Li, 2006; Climent et al., 2018), and liquid - liquid microextraction (He et al., 2015; Shi et al., 2018). The QuEChERS method is a convenient and economical extraction method, and was selected for the present study. Compared with other extraction methods, this method was safe, simple, economical, effective, efficient and reliable. In this study, the QuEChERS method was optimized for extracting and measuring quizalofop-p-ethyl and its metabolite from adzuki bean seeds, plants and soil.
Studying the dissipation dynamics after pesticide application is of great significance in exploring the effectiveness and persistence of the pesticide, and in monitoring the environmental behavior of pesticide (Chen et al., 2018). However, up to now, the residue analysis and dissipation data of quizalofop-p-ethyl and its metabolite in adzuki bean have not been reported. In this paper, improved QuEChERS method was used for sample pretreatment, and HPLC-DAD was used for detection. A field trial was conducted to assess the dissipation dynamics and residue levels of quizalofop-p-ethyl in adzuki bean and soil. This study will provide necessary data for evaluating the safe and effective use of quizalofop-p-ethyl in adzuki bean production.
Chemicals and reagents Standards of quizalofop-p-ethyl (purity >99%) and quizalofop-p-acid (purity >97.6%) were provided by Dr. Ehrenstorfer GmbH (Augsburg, Germany). The molecular structures of the two herbicides are shown in Fig. 1. Commercially available quizalofop-p-ethyl (10% quizalofop-p-ethyl) was purchased from Zhengzhou Dalong Pharmaceutical Company Limited (Henan, China). Analytical reagent grade acetonitrile and ethyl acetate were obtained from Fuyu Fine Chemical Company Limited (Tianjin, China). Analytical-grade reagents dichloromethane, anhydrous magnesium sulfate (MgSO4) and sodium chloride (NaCl) were from Liaoning Quanrui Reagent Company Limited (Liaoning, China). Analytical-grade glacial acetic acid was obtained from Guangfu Technology Development Company Limited (Tianjin, China). Chromatographic grade acetonitrile, methanol, anhydrous magnesium sulfate, and sorbents for dispersive-SPE including primary secondary amine (PSA) and C18 were provided by CNW Technologies Company Limited (Shanghai, China). Pure water was from Hangzhou Wahaha Group Company Limited (Hangzhou, China).

Chemical formulae of quizalofop-p-ethyl (left) and quizalofop-p-acid (right)
Standard solutions Using acetonitrile as solvent, the standard solutions of quizalofop-p-ethyl and quizalofop-p-acid at concentration of 100 mg/mL were prepared and stored in a 4 °C refrigerator. The working standard solution of 10 mg/mL was prepared by diluting the standard solution with acetonitrile. The mixed working standard solutions (0.1, 0.5, 1, 3, 5, 10 µg/mL) were prepared in the same way as the working standard solution and were used to plot the standard curve.
Using the extraction method described in Section 2.5, adzuki bean plants, seeds and soils not treated with herbicide were extracted to obtain blank matrix solution. The matrix standard solutions (0.1, 0.5, 1, 3, 5, 10 µg/mL) were prepared by diluting the standard solutions of quizalofop-p-ethyl and quizalofop-p-acid with blank matrix. The sample concentration was corrected using the matrix curve.
Instrumentation The chromatograph used for the sample analysis was Agilent 1260 Infinity II (Agilent Technologies Incorporated Company, USA). An Athena C18 column (4.6×250 mm, 5 µm, Agilent, USA) was used for chromatographic separation. A detector was G7115A photodiode array detector (DAD) from Agilent. A vortex mixer was Talboys Basic type scroll mixer (Talboys Company, USA). Nitrogen blowing instrument was provided by Tianjin Auto Science Instrument Company Limited (Tianjin, China). Centrifuges were obtained from Changsha Xiangyi Centrifuge Instrument Company Limited (Changsha, China). An electronic balance CP214 was purchased from Ohaus International Trading Company Limited (Shanghai, China). Nylon syringe filters (0.22 mm) were produced by Suzhou Xingkeyuan Information Technology Company Limited (Suzhou, China).
Field trials Field trials were completed in Anda (Heilongjiang province, China, longitude: 125.02, latitude: 46.60) and Zhangjiajie (Hunan province, China, longitude: 110.48, latitude: 29.13). Experiments in Anda and Zhangjiajie began in August and July 2019, respectively. Anda in Heilongjiang province has the temperate continental monsoon climate. Zhangjiajie in Hunan province has the humid subtropical mountain monsoon climate. The soil in the experimental field in Heilongjiang province was Mollisols with organic matter content of 1.93% and pH of 8.44. The soil in the experimental field in Hunan province was Ultisols with organic matter content of 2.44% and pH of 7.86. During the experiment, the average monthly precipitation, average temperature, average humidity, and average monthly sunshine duration in Anda (Heilongjiang) and Zhangjiajie (Hunan) were recorded. In Zhangjiajie city in July, on average precipitation was 151.5 mm, temperature 27.8 °C, humidity 79.4%, and daily sunshine duration was 7 h. In Anda city in August, the average precipitation was 72.7 mm, the average temperature was 22.1 °C, the average humidity was 78.2%, and the sunshine duration was 4.3 h.
The plot design and the experimental procedures followed the agricultural industry standard NY/T788-2004 “Pesticide Residue Test Guidelines and Pesticide Field Test Operating Procedures” of the People's Republic of China. Each test plot covered an area of 30 m2, and there were 3 repetitions. For the final residue tests, adzuki bean plants and soil were treated with the herbicide at 0.3 mL/m2 (the recommended dose), 0.45 mL/m2 (1.5 times the recommended dose) and 0.6 mL/m2 (2 times the recommended dose). Soil, adzuki bean plant and seed samples were collected at harvest time. The reason for high application dose was that weeds developed resistance in many areas, and farmers attempt to effectively remove weeds by increasing the spraying dose (Huan et al., 2011).
The recommended dose of 0.3 mL/m2 was selected for the dissipation dynamic test. The herbicide was sprayed when the bean seedlings grew 1–2 true leaves. Adzuki bean plants and soil samples were randomly collected from the sample plot at 0 (2 h), 1, 2, 3, 5, 7, 14, and 28 days after herbicide spraying. Soil samples were collected at a depth of 10 cm, and stones, plant stems and leaves and other visible material in the soil were removed. After mixing, samples were retained by the quartering method. The collected adzuki bean plants were cut into 1 cm pieces. All samples were stored in a −20 °C refrigerator prior to analysis.
Sample extraction After weighing the homogenized samples, 5 g of adzuki bean plants, seeds or soil samples were loaded into a 50 mL centrifuge tube. Then, 25 mL acetonitrile (containing 1% v/v acetic acid) was added to adzuki bean plant and seed samples. Twenty-five milliliters dichloromethane (containing 1% v/v acetic acid) was added to soil samples. The mixture is then vortexed for 2 minutes, and 0.1 g NaCl and 2.5 g MgSO4 were added to the centrifuge tube, and the mixture was swirled for 1 min followed by centrifugation at 4 000 rpm for 5 min. Then, half of the supernatant was extracted, evaporated under a gentle flow of nitrogen, dried and redissolved in 1 mL of chromatography-grade acetonitrile. The solutions obtained from the redissolved adzuki bean plant and seed samples were transferred to a centrifuge tube (2 mL) containing 150 mg C18 and 50 mg C18, respectively, vortexed for 1 min and centrifuged at 10 000 rpm for 1 min. The supernatant was collected using 1 m L nylon syringe and transferred to the injection bottle through a 0.22 µm filter for HPLC detection.
HPLC conditions The C18 column was placed at 30 °C and the mobile phase was composed of acetonitrile and water (containing 1% v/v acetic acid) = 70:30 (v/v) at a flow rate of 1 mL/min. Twenty microlitesr of the sample was injected into the column and analyzed by high performance liquid chromatography (HPLC). The measurement light wavelength was 236 nm.The external standard peak area was used to quantitatively detect quizalofop-p-ethyl and its metabolite.
Data analysis and calculation Generally, the dissipation dynamics of quizalofop-p-ethyl was fitted to the first-order equation (Eq.1):
 |
where Ct (mg/kg) was the residual concentration of quizalofop-p-ethyl at time t (d), C0 (mg/kg) was the initial concentration, and k was the degradation rate constant (d−1). The half-life was generally expressed by the dissipation time associated with 50% loss (the concentration dropped to 1/2 of the initial concentration) as determined by the formula (Eq. 2):
 |
Optimization of HPLC-DAD method Considering the peak shape, signal strength, sensitivity, and system pressure, acetonitrile/water (containing 1% v/v acetic acid) solution was used as the mobile phase. The elution times of quizalofop-p-ethyl and quizalofop-p-acid were 5.6 min and 13.4 min, respectively, as shown in Fig. 2.

Chromatograms of spiked samples (1 is quizalofop-p-acid and 2 is quizalofop-p-ethyl; Measurement light wavelengths: 236 nm; Injected volume: 20 µL)
Optimization of extraction method Dichloromethane (DCM), methanol (MeOH), ethyl acetate (EtOAc), acetonitrile (ACN), acidulated acetonitrile (containing 1% v/v acetic acid) and acidulated dichloromethane (containing 1% v/v acetic acid) were tested as extractants to optimize recovery efficiency of adzuki bean plants, seeds and soils. As can be seen in Fig. 3, the recovery efficiency of acetonitrile was better than other extractants in the extraction of adzuki bean plants and seeds; moreover, after acetic acid was added to acetonitrile, the recovery efficiency increased significantly. In the extraction of soil samples, the recovery efficiency of dichloromethane regarding quizalofop-p-acid was the best, but it could not meet the standard extraction requirements (>75%). Therefore, 1% acetic acid was added to dichloromethane, and the recovery efficiency of acidulated dichloromethane was increased significantly. Quizalofop-p-ethyl and quizalofop-p-acid belong to acidic pesticides, which are easy to degrade in an alkaline environment, and the addition of acid to the extraction reagent can prevent their loss.

Recoveries of quizalofop-p-ethyl and quizalofop-p-acid with different extractants. Means ±SE (n=3). (Spiked level of quizalofop-p-ethyl: 3 mg/kg; Spiked level of quizalofop-p-acid: 3 mg/kg)
Method validation
Standard curve and limits of quantification Standard curves were drawn based on the concentration (x axis) and peak area (y axis) of quizalofop-p-ethyl and quizalofop-p-acid. The calibration curve is within the operating range of 0.1∼10 µg/mL. The linear equations and the determination coefficient R2 of quizalofop-p-ethyl and quizalofop-p-acid were shown in Table 1.
| Analyte | Regression equation | R2 |
|---|---|---|
| quizalofop-p-ethyl | y = 90.7x + 12.5 | 0.9995 |
| quizalofop-p-acid | y = 117.4x − 9.1 | 0.9998 |
As can be seen in Table 2, the linear regression determination coefficient R2>0.999 was good in different samples (adzuki bean seeds, plants and soils). The limits of detection (LODs) and the limits of quantification (LOQs) were defined as the concentrations corresponding to the signal-to-noise ratio (S/N) of 3 and 10 (Liu et al., 2010), i.e. between 0.003–0.01 mg/kg and 0.01–0.03 mg/kg, respectively.
| Analyte | Matrix | Regression equation | R2 | LOD (mg/kg) | LOQ (mg/kg) | Slope ratio (matrix/solvent) |
|---|---|---|---|---|---|---|
| quizalofop-p-ethyl | seed | y = 92.3x + 3.2 | 0.9993 | 0.005 | 0.015 | 1.02 |
| plants | y = 91.6x + 2.9 | 0.9995 | 0.008 | 0.02 | 1.01 | |
| soil | y = 88.7x + 5.6 | 0.9998 | 0.005 | 0.015 | 0.98 | |
| quizalofop-p-acid | seed | y = 120.2x − 3.6 | 0.9991 | 0.003 | 0.01 | 1.02 |
| plants | y = 119.3x − 6.8 | 0.9997 | 0.01 | 0.03 | 1.02 | |
| soil | y = 99.6x − 8.7 | 0.9996 | 0.003 | 0.01 | 0.85 |
Matrix Effect The composition of the matrix can affect the response of the analyte, and a higher or lower signal can be obtained in the matrix solution than in a standard solvent. The suitability of the quantification method for quizalofop-p-ethyl and quizalofop-p-acid in samples (adzuki bean seeds, plants and soils) was determined by the test method and analyzed by the matrix effect (ME) and standard curve. The matrix effects of all samples were studied by comparing the slope of the matrix matching curve with the calibration curve of the standard solution (Kruve et al., 2008). The results in Table 2 show that matrix effects existed in all substrates, with matrix/solvent slope ratios varying in the range of 85–102%. In adzuki bean seed and plant matrices, the signal intensity of quizalofop-p-ethyl and quizalofop-p-acid increased slightly (ME = 101–102%). In the soil, the signal was weakened compared to solvents (ME = 85–98%). However, the matrix effect was relatively small, and ME was close to 1, suggesting no significant influence on the method.
Accuracy and precision In order to evaluate the precision and accuracy of the method, a recovery experiment was conducted. The unsprayed adzuki bean seeds, plants and soils were each supplemented with quizalofop-p-ethyl at 0.05, 0.2 and 2 mg/kg by adding standard solutions to the samples and left to stand for 1 h to allow the pesticide to penetrate into the samples. The pretreatment and determination were performed by the methods described in Sections 2.5 and 2.6. Measurements were repeated five times a day at each level to obtain intra-day accuracy (repeatability) and tested for inter-day accuracy (reproducibility) over five consecutive days. The precision of the method was indicated by the relative standard deviation between the measured concentrations (RSD). As can be seen in Table 3, the recoveries of quizalofop-p-ethyl and quizalofop-p-acid were 88.7–116.2%, and RSDs were 0.82–4.39%.
| Added (mg/kg) | Intra-day (n=5) | Inter-day (n=5) | ||||
|---|---|---|---|---|---|---|
| Analyte | Matrix | Recovery (%) | RSD (%) | Recovery (%) | RSD (%) | |
| quizalofop-p-ethyl | seed | 0.05 | 98.1 | 2.31 | 96.3 | 3.36 |
| 0.2 | 103.5 | 1.23 | 102.4 | 2.56 | ||
| 2 | 108.3 | 1.32 | 110.6 | 3.21 | ||
| plant | 0.05 | 96.8 | 3.15 | 100.3 | 2.31 | |
| 0.2 | 103.2 | 1.98 | 99.8 | 3.65 | ||
| 2 | 116.2 | 0.82 | 112.9 | 1.52 | ||
| soil | 0.05 | 97.5 | 4.32 | 95.9 | 2.67 | |
| 0.2 | 100.5 | 2.02 | 99.1 | 3.11 | ||
| 2 | 116.2 | 0.82 | 112.9 | 1.52 | ||
| quizalofop-p-acid | seed | 0.05 | 97.9 | 1.99 | 96.7 | 3.61 |
| 0.2 | 102.9 | 2.78 | 106.7 | 2.15 | ||
| 2 | 105.8 | 1.37 | 101.7 | 3.67 | ||
| plant | 0.05 | 99.2 | 2.97 | 102.1 | 1.39 | |
| 0.2 | 101.9 | 1.28 | 102.3 | 0.96 | ||
| 2 | 103.0 | 2.13 | 101.7 | 1.21 | ||
| soil | 0.05 | 89.2 | 3.67 | 91.5 | 4.39 | |
| 0.2 | 92.9 | 2.98 | 90.7 | 3.94 | ||
| 2 | 91.0 | 1.12 | 88.7 | 2.34 | ||
Comparison of the present method with HPLC-MS/MS method At present, few detection methods of quizalofop-p-acid have been reported, so only common detection method of quizalofop-p-ethyl has been compared. Compared with HPLC-MS/MS detection method for the determination of quizalofop-p-ethyl, the present method can still meet the detection requirement (<0.05 mg/kg) although LOQs of present method are higher than those of HPLC-MS/MS method in Table 4. And in terms of economic efficiency, the cost of HPLC-DAD is lower.
| Method | LOQs (mg/kg) | Solvent volumes (mL) | Extraction time (min) | Cost | References |
|---|---|---|---|---|---|
| HPLC-MS/MS | 0.001–0.005 | 15 | 2 | High | Guan and Zhang, 2013 |
| HPLC-DAD | 0.015–0.02 | 15 | 2 | Low | This work |
Dissipation dynamics
Determination of dissipation dynamics and half-life in plants According to the dynamics test method for the dissipation of quizalofop-p-ethyl and its metabolite in adzuki bean plants described in Section 2.4, the herbicide was applied, samples were collected, and the residue was determined. The first-order kinetic equation of quizalofop-p-ethyl residue in the plant was established, and the quizalofop-p-ethyl dissipation curve was obtained (Fig. 4). As can be seen in Fig. 4, the dynamic equation of quizalofop-p-ethyl in the adzuki bean plants from the Heilongjiang test site was Ct = 5.1289e−0.15t. The dynamic equation of quizalofop-p-ethyl in the adzuki bean plants from the Hunan test site was Ct = 3.2919e−0.203t. During the test of dissipation dynamics, the climate conditions and temperature were different at the two sites. Temperatures in Heilongjiang were lower than in Hunan.

Dissipation curves of quizalofop-p-ethyl and quizalofop-p-acid in plants
According to the regression equations, the half-lives of quizalofop-p-ethyl in adzuki bean plants from Heilongjiang and Hunan provinces were 4.6 d and 3.4 d, respectively. The initial concentration of quizalofop-p-ethyl was 9.8 mg/kg in adzuki bean plants from Heilongjiang and 6.9 mg/kg from Hunan. The higher concentration in plants from Heilongjiang province might have been caused by different planting density or uneven spraying. The half-life of quizalofop-p-ethyl was 1.2 d shorter in plants from Hunan than Heilongjiang, likely because of different environments. In the field experiment, the averages of annual sunshine, daily temperature and precipitation were higher in Hunan than Heilongjiang. At 14 d, the dissipation rate of quizalofop-p-ethyl in the plants reached 93.9% (Heilongjiang) and 94.0% (Hunan).
As can be seen in Fig. 4, quizalofop-p-acid in plants from Heilongjiang and Hunan increased to the maximum value from 2 h to 1 d, indicating that quizalofop-p-ethyl decomposed into quizalofop-p-acid. After 1 d, the residual amount decreased with time, and the dynamics of residue diminishing after 1 d was similar to that of quizalofop-p-ethyl.
Determination of dissipation dynamics and half-life in soil According to the test method for the dynamics of dissipation of quizalofop-p-ethyl and its metabolite in soil described in Section 2.4, the herbicide was applied, samples were collected, and the residue was determined. The first-order kinetic equation of quizalofop-p-ethyl residue in the soil was established, and the quizalofop-p-ethyl dissipation curve was obtained (Fig. 5). As can be seen in Fig. 5, the equation of dynamics of dissipation of quizalofop-p-ethyl in the soil from the experimental site of adzuki bean in Heilongjiang was Ct = 0.4761e−0.103t, and the half-life was 6.7 d. The equation of dynamics of dissipation of quizalofop-p-ethyl in the soil from the experimental site of adzuki bean in Hunan was Ct = 0.3186e−0.128t, and the half-life was 5.4 d. The initial concentrations in the soils were 0.9 mg/kg and 0.5 mg/kg in Heilongjiang and Hunan, respectively. The half-life of quizalofop-p-ethyl was shorter in Hunan soil than Heilongjiang soil, indicating faster degradation in the former. This might have been related to the differences in soil water content, organic matter content, pH value, and other soil properties. The organic matter as well as water content were higher in the Hunan soil than Heilongjiang, which might have caused faster dissipation of quizalofop-p-ethyl in the former soil. At 14 d, the dissipation rate of quizalofop-p-ethyl reached 88.8% (Heilongjiang soil) and 92.1% (Hunan soil).
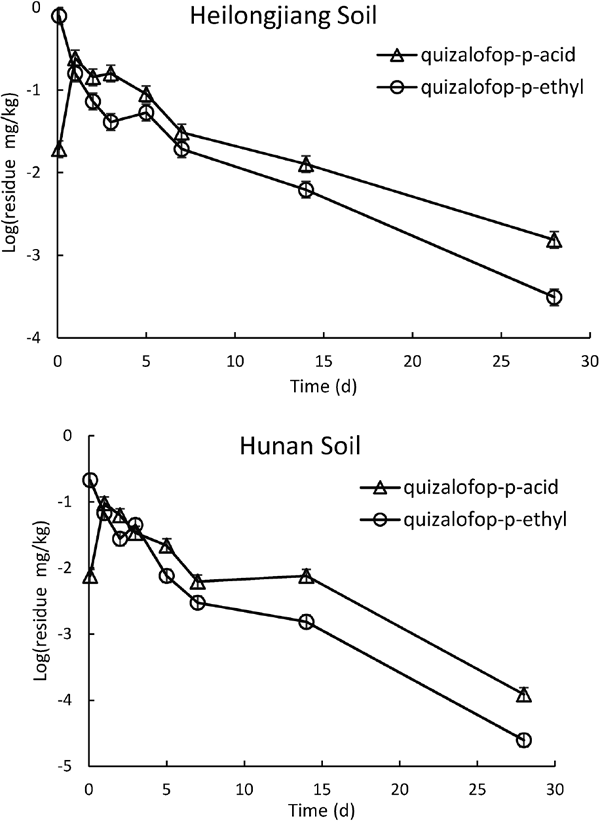
Dissipation curves of quizalofop-p-ethyl and quizalofop-p-acid in soils
There was an increase in quizalofop-p-acid in Heilongjiang and Hunan after spraying, indicating that quizalofop-p-ethyl was decomposed into quizalofop-p-acid (Fig. 5). The quizalofop-p-acid was also decomposed gradually with time, reaching its maximum value about 1 d after spraying. After 1 d, the dissipation dynamics of quizalofop-p-acid in soil was similar to that of quizalofop-p-ethyl.
Terminal residues In the test of quizalofop-p-ethyl residues in adzuki bean, the low dose 0.3 mL/m2 (recommended dose), medium dose 0.45 mL/m2 (1.5 times the recommended dose) and high dose 0.6 mL/m2 (2 times the recommended dose) were sprayed evenly on adzuki bean plants and soils. Adzuki bean seed, plant and soil samples were collected at harvest time to determine the final residue. At harvest time (Heilongjiang province in October 2019, Hunan province in September 2019), the final residue of quizalofop-p-ethyl and its metabolite in adzuki bean seed, plants and soil from Hunan and Heilongjiang was less than LOQ. The maximum permissible residue limit (MRL) established by the USA Department of Agriculture was 0.05 mg/kg, whereas the maximum permissible residue limit established by the Chinese Ministry of Agriculture was 0.1 mg/kg. The present study indicated that the herbicide was safe to use at the recommended dose.
In this study, we established a simple, efficient, rapid, low-cost, and reliable method for determination of herbicide residues. HPLC-DAD was used to simultaneously determine quizalofop-p-ethyl and its metabolite in adzuki bean seeds, plants and soil. The detection method had good linear regression, R2>0.999. After spiking, the recoveries of quizalofop-p-ethyl and quizalofop-p-acid were high, ranging from 88.7 to 116.2%. The method was reliable and stable, with RSDs between 0.82 and 4.39%. The LODs and LOQs were 0.003–0.01 mg/kg and 0.01–0.03 mg/kg respectively, which met the detection requirements.
The dissipation dynamics of quizalofop-p-ethyl in the adzuki bean plants and soils conformed to the first-order model Ct=C0e−kt. The half-life of quizalofop-p-ethyl in the plants was 3.4–4.6 d. The half-life in soil was 5.4–6.7 d. After spraying the adzuki bean plants and soil with low herbicide dose of 0.3 mL/m2 (recommended dose), medium dose of 0.45 mL/m2 (1.5 times the recommended dose) and high dose of 0.6 mL/m2 (2 times the recommended dose), the final residue of quizalofop-p-ethyl and its metabolite in adzuki bean plants, seeds and soil were all <LOQ at harvest.
Quizalofop-p-ethyl was dissipated in adzuki bean plants and soils from Heilongjiang more slowly compared with Hunan. The residues of quizalofop-p-ethyl and its metabolite in plants and soils were lower than the MRL, indicating it is safe to apply adzuki bean at the recommended dose. This work is important for the Chinese government to (i) establish the residue analysis standard of quizalofop-p-ethyl in adzuki bean and (ii) guide the rational and safe use of quizalofop-p-ethyl in adzuki bean.
Acknowledgements This study was funded by Department of Education, Heilongjiang Province (grant number [2018] No. 4). This study was funded by the National Key R&D Program of China (2018YFE0206300 and 2018YFE0206300-10). This study was funded by Heilongjiang Bayi Agricultural University (TDJH201806)