2020 Volume 26 Issue 5 Pages 655-663
2020 Volume 26 Issue 5 Pages 655-663
Defatted sesame meal (DSM) were explored as a stabilizer in soybean-oil-in-water (O/W) emulsions. The major parameters that affect the stability of emulsions were investigated. The suitable condition for the preparation of O/W emulsions stabilized with DSM were 3.0 wt% of DSM, 40% of soybean oil and pH value at 4.0. Rheological behavior of the emulsion showed that the emulsions had pseudo-plastic fluid characteristics. The elastic modulus (G′) was always higher than loss modulus (G″) at frequencies of 0.1–10 Hz. Taken together these results suggested that an inter-droplet network formed resulting in a gel-like behavior of the emulsion. This is first study to characterize DSM as a stabilizer in O/W emulsion to date and may provide guidance for maximizing the utilization of sesame in the industry.
Sesame seed contains up to 50% lipids. Around 70% of sesame produced all over the world are used for oil extraction (Islam et al., 2016). The residual from sesame oil extraction is known as defatted sesame meal (DSM). It contains protein (> 40%), carbohydrate (10%–20%) and other nutritional components (Namiki, 2007). In industry it is mainly used as fertilizer or animal feed (Obeidat et al., 2009), or disposed as wastes. As researchers pay attention to the sustainable development of industrial and agricultural by-products, the deep processing technology of sesame meal has caused the extensive interest in enhancing the added value of the sesame meal. At present, further processing of sesame meal mainly centralizes on the extraction of bioactive constituents, including sesame proteins (Chatterjee et al., 2015), sesame bioactive peptides (Chatterjee et al., 2015), sesamol (Jeon et al., 2016), sesamin (Jeon et al., 2016) and lignans crude extracts (Ben Othman et al., 2015). These processes are laborious and have not utilized the sesame meal to a full extent.
Recently Joshi et al. (2015) have reported that emulsifying capacity and gelation properties of defatted sesame flour are higher than those of defatted soy flour and peanut flour. Additionally, the water and oil holding capacity are higher than those of defatted hazelnut flour. Turan et al. (2015) have reported that the roasted defatted hazelnut flour without further modification could stabilize the emulsion. Previous studies have shown that protein isolates from sesame have emulsifying properties (Brewer et al., 2016; Sharma et al., 2016). However, to date the exploration of DSM as a stabilizer has not yet been reported. The investigation of DSM as a stabilizer may provide an economic alternate for food-grade emulsifier from animals and maximize the utilization of residues of sesame after oil extraction.
Therefore, the purpose of presented study is to investigate DSM as a natural emulsion stabilizers. DSM was prepared by grinding, defatting, drying and sieving through 40-mesh sieves. Then composition and interfacial property were analyzed. Afterwards DSM was applied to stabilize soybean oil-water emulsion. The droplet size, microstructure, stability and rheological properties of the emulsion were characterized. The exploration of DSM as an economic food-grade emulsifier of natural origin will facilitate the sustainable development of sesame in industry and the utilization of resources to the maximum extend.
Materials White sesame seeds and soybean oil were purchased from a local supermarket in Wuhan (P. R. China). Other chemicals in the study were of analytical grade and obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, P. R. China). Deionized water was used for experiments.
Defatted sesame meal (DSM) preparation Raw sesame seeds were ground using a mini-type herbal medicine grinding machines (FW135 Tianjin, P.R. China) at a speed of 24 000 rpm to obtain full fat flour. Then the full fat flour was mixed with petroleum ether (boiling point range of 30 °C −60 °C) with a ratio of 1/5 (wt/wt) and the mixture was placed in a 30 °C shaking bed for 48 h at a speed of 150 rpm to remove fat. The defatted meal was firstly dried at room temperature and then in an oven at 60 °C for 4 h. Afterwards the sesame meal was ground again to pass through a 40-mesh sieve. As-prepared DSM was sealed in polyethylene bags and stored in the drier at room temperature for further analyses.
Composition analyses of DSM Total protein content of DSM was evaluated using Kjeldahl method with a Hanon nitrogen analyzer and a conversion factor of 6.25 (Amza et al., 2011). Lipid, moisture and ash contents were determined using standard AOAC methods (Helrich, 1990). Total carbohydrate content was calculated as follows:
 |
Interfacial tension measurements of DSM Interfacial tension was determined as previously reported with minor modifications (Turan et al., 2015; Yang et al., 2013). The equilibrium interfacial tension between water and soybean oil surface was determined with a tensiometer (DCAT11EC, DataPhysics, Germany) at 25 °C using the Wilhelm plate method. At first surface tension of 45 mL soybean oil was quantified. Then 45 mL of soybean oil was carefully poured onto the top of 30 mL of aqueous solution of DSM in the vessel. Afterwards the mixture was equilibrated to the measurement temperature and the surface tension was recorded. The precision of the interfacial tension measurements was 10−5 mN/m.
Emulsion preparation O/W emulsion was prepared according to previously reported methods (Liu and Tang, 2014). Firstly, DSM dispersion was prepared by adding DSM to 0.02% (w/v) of sodium azide aqueous solution. The sample was then homogenized under high mechanical shear with an UltraTurrax®T25 equipped with a dispersing tool consisting of S25N-18G shaft (IKA, Germany) at 10 000 rpm for 5 min at room temperature. Afterwards DSM dispersion was blended with different ratio of soybean oil to form O/W emulsion with the homogenizer at 10 000 rpm for 5 min again, and at 80 MPa for 4 passes using high-pressure homogenizer (AH-2010, ATS Engineering Inc., Canada). The emulsion was prepared and stored at room temperature for 24 h before analyses.
Droplet size determination of the emulsion The mean particle sizes of droplets in emulsions after being freshly prepared or stored for indicated periods (7–30 days) was examined using MasterSizer 2000 (Malvern, UK). Deionized water was used as the dispersant. The particle size was reported as area-average size, d3,2=(∑nidi3/∑nidi2), where ni was the number of droplets with diameter di. Measurements were performed at 25 °C and conducted for three times.
ζ-potential measurements of the emulsion droplets ζ-potentials of droplets in emulsions was measured with a Zetasizer NanoZS90 (Malvern Instruments, UK) at 25 °C. All samples were diluted to maintain 0.5% of oil content. Mean values and standard deviations of three replicates were reported.
Visual observation of creaming and determination of creaming index Creaming stability of emulsions was evaluated as previously reported (Hu et al., 2016; Liu and Tang, 2013). Briefly 10 mL of each emulsion was transferred into a glass tube and stored at room temperature in a perpendicular state. During the storage up to 2 months the volume of serum (Vs) and total volume of emulsion (Vt) at indicated interval were recorded. The percentage of creaming index (CI%) was calculated as (1−Vs /Vt) × 100. Mean values and standard deviations of three replicates were reported.
Optical microscopy observation of the emulsion The microstructure of freshly prepared and stored emulsion was analyzed with optical microscopy (WV-CP470, Panasonic, Japan) at different magnifications of water-immersion objective. Ten microliters of emulsions with appropriate dilutions were directly placed on a glass slide with a coverslip on the top for the microscopic observation.
Rheological measurements Rheological properties of emulsions were characterized as previously reported (Derkach et al., 2015) with minor modification. The measurements were performed using a controlled stress rheometer (AR2000ex, TA, USA) with a parallel-plate geometry (40 mm diameter, 1 mm gap) at 25 °C. Steady-shear rate flow curves were set from 0.01 to 100 s−1. The linear viscoelastic regime measurement was performed at 0.2% strain and oscillatory frequency sweep tests ranged from 0.1 to 10 Hz.
Statistical analyses All experiments and analyses were performed in triplicates. Data were expressed as means ± standard deviation. The average values were compared by one-way analysis of variance (ANOVA) with t test using SigmaPlot10.0 software (Systat Software, Inc., Chicago, USA). The significant difference among means was estimated at 95% confidence level (p < 0.05).
Composition of DSM The composition of defatted sesame meal (DSM) was summarized in Table 1. As expected, the extraction of oil from sesame seed resulted in an increase of percentages of other constituents in DSM. Among them, protein content was increased from 24.8% in the sesame seed to 63% in DSM, which was close to the content of sesame protein concentration reported elsewhere (Inyang and Iduh, 1996). Besides DSM consisted of 19.2% carbohydrates, 6.13% ash and 4.62% crude fiber. Although DSM was defatted with the petroleum ether for three times, there was a small amount of fat residual (1.63%).
| DSM | Content (g/100 g) |
|---|---|
| Crude Protein (%) | 63.18 ± 5.87a |
| Carbohydrate (%) | 19.72 ± 1.54b |
| Crude Fat (%) | 1.68 ± 0.28c |
| Ash (%) | 6.13 ± 0.55cd |
| Water (%) | 9.24 ± 0.76de |
Means ± standard error. Different letters (a, b, c, d) in the same row were signi.cantly different, p < 0.05.
Impact of DSM concentration on the interfacial tension The impact of DSM concentration on the interfacial tension of soybean oil-water surface was given in Table 2. DSM were dispersed in aqueous solution at the concentration of 1.0%, 2.0%, 3.0% and 4.0% without addition of salts at neutral pH value, afterwards soybean oil was poured onto the top of DSM dispersion. The equilibrium interfacial tensions and the adsorption equilibrium time between water and soybean oil surface were measured. According to Table 2, both the equilibrium interfacial tension value and the adsorption equilibrium time were decreased upon with the increase of DSM concentration, which demonstrated that DSM was rapidly absorbed to the oil-water interface. This may be the reason that a higher concentration of DSM possessed more numerous amphiphilic molecules and more dispersed in the interface, which truncated the distance to the oil-water interface.
| DSM concentration | |||||
|---|---|---|---|---|---|
| 0% | 1% | 2% | 3% | 4% | |
| Interfacial tension (mN/m) | 18.35 ± 0.17 | 8.13 ± 0.08 | 2.47 ± 0.17 | 1.77 ± 0.18 | 0.98 ± 0.16 |
| Adsorption equilibrium time (s) | 9933 | 8506 | 4997 | 3527 | 2939 |
It has been reported that the interfacial tension of classical surfactant Tween 80 at concentration of 1.0% was about 5 mN/m (Yang et al., 2013). The interfacial tension of pure soybean-water interface was 18.35 mN/m (Table 2), DSM at a concentration of 2.0% significantly reduced the interfacial tension of emulsions down to 2.47 mN/m and 4.0% of DSM reduced the interfacial tension value to 0.98 mN/m. The excellent performance of DSM in the reduction of interfacial tension indicates that DSM have a potential application as an emulsifying stabilizer.
Impact of DSM concentration on the formation of emulsions DSM-stabilized O/W emulsion were prepared with varied DSM concentrations and oil fraction (φ) of 0.4 at neutral pH value. The impact of DSM concentrations including 1.0%, 2.0%, 2.5%, 3.0%, 3.5% and 4.0% in ultra-pure water on the droplet size of the emulsion was investigated. Fig. 1a showed typical droplet size distribution of freshly prepared emulsion and Fig. 1b showed area-average diameter data after storage at room temperature for 0–30 days as a function of c. According to Fig. 1b, d3,2 values of O/W emulsions stabilized with 1.0%, 2.0%, 2.5%, 3.0%, 3.5% and 4.0% of DSM were 39.2, 23.3, 18.4, 15.2, 13.0 and 12.7 µm respectively. The droplet size of the emulsions gradually decreased upon the increase of DSM concentrations, which usually represented an increase in interfacial area and was in line with the fact that higher particle content facilitated the stabilization of larger interfacial area.

Impact of DSM concentration on (a) the size distribution of freshly-prepared emulsions in presence of DSM at indicated concentration, (b) d3,2 of DSM-stabilized emulsions after storage for 0, 7, 14, 21 and 30 days, (c) the creaming index (CI%) over the storage time and (d) creaming volume after equilibrium by visual observation.
Moreover, the creaming stability expressed as the percentage of creaming index (CI%) during storage up to 60 days was monitored. The data and the image were presented in Fig. 1c and Fig. 1d respectively. In general CI% of all emulsions increased promptly within approximately 1 h of storage, followed by a slow increase. After three and more days, CI% remained almost unchanged. In the steady state of creamed emulsions, the increase of c from 1.0 to 4.0% resulted in a progressive decline in CI%, which indicated that the increase of DSM concentration resulted in the inhibition of creaming. Similar phenomena had been observed for the emulsion stabilized with SPI aggregates (Liu and Tang, 2013), MCC (Xhanari et al., 2011) and chitin nanocrystals (Tzoumaki et al., 2011).
Furthermore, the impact of DSM concentration on the rheological properties of the emulsion was investigated and displayed in Fig. 2a–c. The measurement of frequency-dependent linear interfacial viscoelastic moduli (G′ and G″) was performed and plotted in Fig. 2a and b. All the emulsions showed a higher G′ than G″ within the range of 0.1–10 Hz. G′ was increased with the increase of DSM concentration from 1.0% to 4.0%. However, G′ of emulsion with DSM at the concentration of 3.5% and 4.0% was not significantly higher than that with DSM at the concentration of 3.0%. In the presence of DSM from 1.0% to 4.0%, shear viscosity of the emulsion in Fig. 2c was rapidly decreased upon the increase of shear rate and it displayed a pseudo plastic fluid behavior. Meanwhile viscosity was enhanced with the increase of DSM concentration and was mostly pronounced with DSM of 3.0 wt% than that of 4.0 wt%. It indicated that a weak network structure of droplets was formed (Song et al., 2015; Xiao et al., 2016). Taken together the results indicated the essential role of DSM concentration in the structure formation of emulsions and DSM-stabilized emulsion showed a gel-like behavior. The formation of a gel-like network among flocculated oil droplets suppressed the size increase and creaming after the emulsion being stored for 30 days. Thus, the suitable DSM concentration was selected as 3.0% (wt/wt).

Impact of DSM concentration on the (a) elastic modulus, (b) viscous modulus and (c) complex viscosity of the emulsion.
Impact of oil fraction (φ) on the formation of emulsions The impact of oil fraction on the stability of emulsion was investigated and shown in Fig. 3. DSM was set at concentration of 3.0%, and the emulsion was prepared with soybean oil fractions ranging from 0.2 to 0.6 at neutral pH value. The size distribution of various fresh emulsion was shown in Fig. 3a. According to Fig. 3a, oil droplets with the amount of DSM at above 0.5 became not stable and the size peak of droplets became large. Interestingly, the droplet size of the emulsion decreased with the increase of φ from 0.2 to 0.4; d3,2 of emulsion droplets progressively declined from about 18 to 13 µm (Fig. 3b). Additionally, no obvious increase of droplet size was observed in the emulsion with φ ranging from 0.2 to 0.5 after being stored at room temperature for 7 days. However, the droplet size of the emulsion with oil fraction of 0.6 sharply increased and the oil precipitated from the emulsion, which was displayed in Fig. 3b and Fig. 3d. The impact of oil fraction on creaming stability of emulsion was shown in Fig. 3c and the appearance after storage at room temperature for 60 days was shown in Fig. 3d. All of emulsions except for the one with oil fraction of 0.6 showed excellent long-term stability after 60 days of storage and the creamed contents decreased with the increase of φ from 0.2 to 0.5. The reason was that high content of oil forming a gel-like network in the emulsion hindered the formation of O/W cream and the oil droplet acted as filling agent in the network (Tang and Liu, 2013).
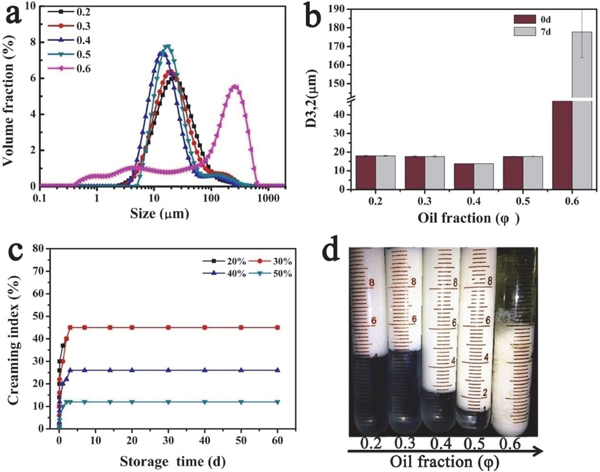
Impact of oil fraction on (a) the size distribution of freshly-prepared emulsions, (b) d3,2 of freshly-prepared emulsions and the emulsion after storage for 7 d, (c) creaming index (CI%) and (d) creaming after equilibrium by visual observation.
Fig. 4 (a–c) showed the impact of oil fraction on the viscoelastic properties of emulsions. G′ (Fig. 4a) was much larger than G″ (Fig. 4b) in the entire frequency range, suggesting that the behavior of the emulsion was predominantly elastic and a three-dimensional network formed in the emulsion. As the increase of φ from 0.2 to 0.5, the values of G′ and G″ were gradually aggrandized but their incremental rate was decreased upon the increase of oil fraction. For instance, at the frequency of one, φ increased from 0.2 to 0.3, G′ and G″ were improved by 53.2 Pa and 17.4 Pa respectively; φ increased from 0.4 to 0.5, G′ and G″ were increased by 13.8 Pa and 2.0 Pa respectively. It turned out that a higher oil phase could form more compact gel network structure and hinder the oil droplets from flocculation or aggregation, which was consistent with the behavior of Pickering emulsion stabilized with protein nanoparticles (Xiao et al., 2016). At the same time, the initial viscosity was larger with the increase of oil fraction, as shown in Fig. 4c. According to Fig. 4c, viscosities of the emulsion increased with an increase of oil fraction, and this resulted in less cream volumes (Fig. 3d). This was consistent with the previous report (Tang and Liu, 2013), upon the increase of oil fraction the gel network became more tight by padding with oil droplets which led to the increase of viscosity. However, when oil fraction was increased from 0.4 to 0.5, the viscosity change of the emulsion was not significant. Thus, the suitable soybean oil fraction was selected as 0.4.

Impact of oil fraction on the (a) elastic modulus, (b) viscous modulus and (c) complex viscosity of emulsions.
Impact of ionic strength on the stability of emulsions The addition of NaCl from 0 to 500 mM on the stability of DSM dispersion in ultra-pure water and O/W emulsions was investigated and analyzed in aspects of droplet size, zeta potential and creaming index (CI%). DSM concentration and oil fraction were set at 3.0% and 0.4, respectively. The results were exhibited in Fig. 5. The droplet size of emulsions was not significantly changed in the presence of NaCl (Fig. 5a), but the creaming volume of the emulsion via visual observation was markedly increased (Fig. 5d) with an increase of NaCl concentration. Because of the electrostatic screening caused by the addition of NaCl, increased ionic strength from 0 to 500 mM resulted in a progressive decline (up to −4.5 mV) of ζ-potential (Fig. 5b). Higher interparticle electrostatic repulsion caused higher creaming volume (Fig. 5c). Although flocculation occurred, no precipitation was observed in the presence of high concentration of salts.

Impact of NaCl on the stability of emulsions. (a) Droplet size of freshly-prepared emulsions in presence of NaCl at indicated concentration; (b) Zeta potential of DSM and emulsion in presence of NaCl at indicated concentration; (c) Creaming index (CI%) of emulsions in presence of NaCl at indicated concentration over the storage duration; (d) Visual observation of creaming after equilibrium in presence of NaCl at indicated concentration.
Impact of pH on the stability of emulsions As it was displayed in Table 1, the main composition of DSM was sesame protein. Since the emulsifying property of protein depended on pH value (Inyang and Iduh, 1996), the impact of pH on the stability of emulsion stabilized with DSM was essential. DSM-stabilized O/W emulsion was prepared with 3.0% of DSM in ultra-pure water and 0.4 of oil fraction. Afterwards droplet size, zeta potential, creaming volume and creaming index were monitored under pH value ranging from 4 to 9. As it was shown in Fig. 6a, the droplet size of emulsions was smallest at pH 7.0. With the pH value below or above 7.0, the emulsion droplets had an increased size. The size of the emulsion reached biggest at pH value of 9.0. According to Fig. 6b, zeta potential of emulsions shared the similar trend with DSM aqueous solution that they increased upon the increase of pH value from 4.0 to 9.0. The creaming volume was largest at pH 4.0 (Fig. 6d) and CI% (Fig. 6c) was lower than 2% after the storage of 2 months due to the high viscosity, suggesting the suitable pH value for the preparation of emulsion was 4.0. Several studies have reported that protein-polysaccharide conjugate can markedly enhance emulsion stabilization (Neirynck et al., 2004). A recent study has reported that the introduction of maltodextrin into sesame protein concentrate during conjugation enhanced the solubility of conjugated protein at isoelectric point and steric stabilization provided by the bulky hydrophilic polysaccharide moiety, thereby improving emulsifying properties of sesame protein. In our study, major composition of DSM was protein which took part up to 63% of the total content and the second most abundant composition of DSM was carbohydrate which was 20% of the whole content (Table 1). The improvement of emulsifying stability at pH 4 (isoelectric point) of DSM was assumed to be resulted from the interaction of proteins and carbohydrates in the meal (Saatchi et al., 2019).

Impact of pH on the stability of emulsions. (a) Droplet size of freshly-prepared emulsions at various pH value; (b) Zeta potential of DSM and emulsion at various pH value; (c) Creaming index (CI%) of emulsions at various pH value over the storage duration; (d) Visual observation of creaming after equilibrium at various pH value.
DSM was a residue from the oil extraction of sesame and was explored as a novel food stabilizer in presented study. The emulsions stabilized with DSM exhibited similar physicochemical and microstructure characteristics of those stabilized with well-known Pickering stabilizers in previous reports. The increase of DSM concentrations resulted in the smaller droplet size of emulsions and the higher stability against coalescence or creaming. With the addition of 3.0% of DSM, the increase of oil fraction within the range of 0.2–0.5 facilitated the formation of gel-like emulsions that had a smaller size of particles and better creaming stability. This was the first study to date to develop DSM as an effective stabilizer in O/W emulsion, which was assumed to promote the utilization of sesame in the industry.
Acknowledgments The work was financially supported by Natural Science Foundation of Hubei Province (2018CFB379) and Fundamental Research Funds for the Central Universities (2662017QD006).