2020 年 26 巻 6 号 p. 779-787
2020 年 26 巻 6 号 p. 779-787
The anti-hypertensive effects of hesperidin and hesperidin-containing fermented Mikan tea were investigated in 8-week-old spontaneously hypertensive rats (SHRs) at a dose of 50 mg/kg body weight for both, over 20 weeks. At the end of the protocol, a significant (P < 0.05) reduction in the systolic blood pressure (SBP) of approximately 60 mmHg was observed in the SHRs with the intake of hesperidin and Mikan tea. No change in the heart rate was observed between groups. Hesperidin altered the impaired vasomotor response in 28-week-old SHRs. Investigation of receptor expression related to mitogen-stimulated vasomotor action revealed that the Mas receptor (MasR) in the aorta of hesperidin-administered SHRs was up-regulated, while no changes in angiotensin II-type 1 and type 2 receptor expressions were observed. An increase in cAMP levels was confirmed in the aorta of hesperidin-administered SHRs, demonstrating that anti-hypertensive hesperidin plays a role in vessel regulation via the upregulated-MasR/cAMP axis.
Naturally occurring polyphenols have been extensively investigated due to their numerous health benefits to humans (Pandey and Rizvi, 2009). Epidemiological studies have revealed that the consumption of polyphenols is effective for preventing hypertension, osteoporosis, neurodegenerative diseases, diabetes, and obesity (Cory et al., 2018). Taking into consideration such beneficial effects resulting from the efficient intake of polyphenols, we have formulated a new fermented Mikan tea, consisting of third-crop green tea (Camellia sinensis) leaves and unripe satsuma mandarin (Citrus unshiu) fruit (Nakayama et al., 2014), in which soluble condensed catechins (e.g., theaflavins and theasinensins) and insoluble polyphenols (e.g., hesperidin) are co-present. The co-present polyphenols in Mikan tea enable the solubilization of insoluble hesperidin, by formation of stable complexes between the condensed catechins in the fermented tea and hesperidin (Cao et al., 2015). Additionally, in our previous human study of Mikan tea by a randomized, double-blind, placebo-controlled, parallel group comparative test, elevated systolic blood pressures (SBP) of 20 high-normal and mildly hypertensive volunteers were significantly ameliorated following the daily intake of Mikan tea (890 mg/day) for 12 weeks (ΔSBP, ca. 8 mmHg) (K. Tanaka et al., 2020). However, the underlying anti-hypertensive mechanism(s) remains unclear.
BP is determined by fluid volume and vessel resistance. Thus, the renin-angiotensin system (RAS) is thought to be closely associated with the onset of hypertension, as a pressor hormone present in the system, angiotensin (Ang) II, stimulates both BP determining factors. Thus far, it has been reported that hesperidin reduces elevated vessel resistance by promoting NO production (Wunpathe et al., 2018) or by inhibiting NADPH oxidase (Yamamoto et al., 2013), resulting in a reduction in BP in spontaneously hypertensive rats (SHRs) (Ohtsuki et al., 2002) and in humans (Morand et al., 2011). In this study, the physiological mechanism of Mikan tea or hesperidin action in vessels was investigated.
Our recent report demonstrated the absorption of hesperidin in its intact and conjugated forms, as well as of its aglycone (hesperetin), in rat bloodstream (Nectoux et al., 2019). Taken together, the aim of this study was the evaluation of in vivo anti-hypertensive effects of absorbable hesperidin and hesperidin-containing Mikan tea in SHRs following their long-term administration. We additionally investigated the mechanisms giving rise to the anti-hypertensive effect by focusing on the circulating and local RAS.
Materials Mikan tea was prepared according to our previous report (Nakayama et al., 2015). Briefly, third-crop green tea (Camellia sinensis) leaves and unripe satsuma mandarin (Citrus unshiu) fruit were mixed in a ratio of 3:1, followed by kneading with a tea roller (120 k-2 type, Kawasaki Co.) at 26 °C for 20 min. A hot-water extract of Mikan tea was used for the administration experiments, wherein 1 L of hot water was added to 20 g of Mikan tea product and the tea was stirred for 10 min. The supernatant was then lyophilized to obtain the extract powder (5.6 g), containing 62.7 mg of hesperidin. Hesperidin (hesperetin-7-O-rutinoside) was purchased from Funakoshi Co. (Tokyo, Japan). Phenylephrine (PE) and acetylcholine (ACh) were acquired from Wako Pure Chemical Ind. (Osaka, Japan). Anti-Ang II type 1 receptor (AT1R) primary antibody (ab124505, Lot: GR3188953-21) and anti-Ang II type 2 receptor (AT2R) primary antibody (ab92445, Lot: GR109798-31) were purchased from Abcam (Cambridge, MA, USA). Anti-Mas receptor (MasR) primary antibody (AAR-013, Lot: AAR013AN1102) was obtained from Alomone Labs (Jerusalem, Israel). Rat Ang (1–7) ELISA kit (MBS731540, Lot: 20191216C) was purchased from My Biosource (San Diego, CA, USA). Cyclic AMP (cAMP) ELISA kit (581002, Lot: 0580287) was sourced from Cayman Chemical Co. (Ann Arbor, MI, USA). NOx (NO2/NO3) Assay-FX (fluorometric) kit (NK08, Lot: ND826) was purchased from Dojindo Molecular Technologies, Inc. (Kumamoto, Japan). The Ang II-EIA kit (ADI-900-204, Lot: 06041801C) was procured from Enzo Life Sciences, Inc. (Farmingdale, NY, USA). All other chemicals were of analytical grade and were used without further purification.
Animal experiments Seven-week-old male SHRs were supplied by the Disease Model Cooperative Research Association (SHR/Izm, Shizuoka, Japan). The rats were individually housed for one week at 21 ± 1 °C and 55.5 ± 5% humidity under controlled lighting from 8:30 to 20:30. Rats were fed a laboratory diet (CRF-1 diet, Charles River Co., Kanagawa, Japan) and provided with distilled water ad libitum. Eight-week-old SHRs were randomly divided into three groups (n = 4/group), and oral administration of samples was performed daily for 20 weeks: control group, saline solution; hesperidin group, 50 mg/kg of hesperidin dissolved in saline solution containing 0.5% carboxymethyl cellulose; Mikan group, 50 mg/kg/day (containing 0.56 mg hesperidin/kg/day) dissolved in saline solution. Systolic/diastolic blood pressure (SBP/DBP) and heart rate (HR) were measured successively five times with a non-invasive tail-cuff microsensor device, without warming the rats (model MK-2000A; Muromachi Kikai, Tokyo, Japan) at 8, 10, 12, 14, 16, 18, 20, 23, and 28 weeks of age. An average of three measurements was recorded for each animal after eliminating the highest and lowest BP measurements. During the experimental period, diet intake and body weight (BW) were recorded weekly. All animal experiments were conducted following the guidelines set by the Guidance for Animal Experiments by the Faculty of Agriculture and the Graduate Course of Kyushu University under the Law (no. 105, 1973) and notification (no. 6, 1980, of the Prime Minister's Office) of the Japanese Government. The experimental design was approved by the Animal Care and Use Committee of Kyushu University (permit number: A30-015-2).
Measurement of vasomotor response in isolated aortic rings At 28 weeks of age, all rats were anesthetized by sevoflurane (Maruishi Pharmaceutical Co., Ltd, Osaka, Japan). Blood was collected from the abdominal aorta, and mixed with the anti-coagulant EDTA-2Na, aprotinin, and chymostatin for further biochemical measurements. The thoracic aorta was used for vasomotor response experiments, and the aorta rings were prepared according to our previous study (Fukuda et al., 2014). Briefly, the thoracic aorta from euthanized rats was carefully excised, and dissected in physiological salt solution buffer (PSS, pH 7.4, composition in mM: NaCl 145, KCl 5, Na2HPO4 1, CaCl2 2.5, MgSO4 0.5, glucose 10, and HEPES 5) at 37 °C to remove adhered fat and connective tissue. The vessels were then sliced into rings 2–3 mm in length. The ring segments were mounted on stainless steel hooks within a 5-mL organ bath filled with PSS buffer with 95% O2/5% CO2 gas at 37 °C. The rings were stretched to an equivalent of 2 g tension for 45 min until stabilized. After equilibration, contractions were evaluated with the addition of 1 mM PE for 20 min. Relaxation was monitored by the addition of 100 µM ACh. The relative vasomotor response (contraction/relaxation potential) of a given ring was evaluated by the ratio of reduced tension by ACh to increased tension by PE (Fukuda et al., 2014). The tissue response (isometric tension, in g) was measured using a force transducer (Micro Tissue Organ Bath, Model MTOB-1Z; Labo Support, Osaka, Japan) and a recorder (4-channel amplifier; EMKA Technologies, Paris, France).
Biochemical Measurements Blood samples were centrifuged at 3 500 × g for 15 min at 4 °C to obtain plasma. The concentrations of plasma Ang II, Ang (1–7), and NOx were determined using their corresponding assay kits, according to the manufacturer's instructions. Arterial cAMP in thoracic aorta homogenized with a Polytron homogenizer (25 000 rpm, 20 s, 10 repetitions, 4 °C; Bohemia, NY, USA) was assayed with the cAMP ELISA kit. All measurements were performed with a FlexStation 3 microplate reader (Molecular Devices, Sunnyvale, CA, USA).
Protein expression by Wes analysis Protein levels of AT1R, AT2R, and MasR in aorta were measured with a capillary electrophoretic-based immunoassay Wes instrument (ProteinSimple Co., San Jose, CA, USA). Briefly, the aorta was homogenized with a microtube homogenizer (Bohemia, NY, USA) in a lysis buffer containing PBS with 0.1% Triton X-100, 1% NP-40, 1% protease inhibitor cocktail (Nacalai Tesque Co., Kyoto, Japan) and 1% PhosSTOP (Roche, Indianapolis, IN, USA). The homogenate was centrifuged at 14 000 × g for 15 min at 4 °C, and the supernatant was subjected to Wes measurement. The Wes measurements were conducted with a 12–230 kDa separation module (8 × 25 mm capillary cartridge, ProteinSimple Co.), according to our previous paper (M. Tanaka et al., 2020). For AT1R, AT2R, and MasR detection, the lysates diluted with a 0.1 × Sample Diluent buffer were then mixed with a 5 × Fluorescent Master Mix (ProteinSimple Co.) at a ratio of 4:1. An aliquot (0.5 µg/µL for AT1R and AT2R; 1 µg/µL for MasR) of proteins was applied to the instrument, and then heated for denaturation at 95 °C for 5 min. Wes reagents (biotinylated ladder and primary antibodies) were dispensed in a microplate and subjected to Wes automated capillary electrophoresis, followed by automated immunodetection using a horseradish peroxidase-conjugated anti-rabbit secondary antibody and a chemiluminescent substrate. Primary antibodies for the targeted proteins AT1R, AT2R, and MasR were diluted at 1:100. For total protein detection, a pentafluorophenyl ester-biotin labeling reagent that can attach to applied proteins was used. The resulting chemiluminescent signal was displayed as a virtual blot-like image and the electropherogram was based on the molecular weight using Compass software (ProteinSimple Co.). Protein expression of AT1R, AT2R, and MasR was normalized to the electropherogram peak areas of total protein applied to each lane. The data were expressed as ratios against the control.
Statistical analysis Results were expressed as the mean ± standard error of the mean (SEM). All analyses were performed using GraphPad Prism software (La Jolla, CA, USA). Statistical differences between the two groups were evaluated using Student's t-test. A P value < 0.05 was considered significant.
Effects of hesperidin or Mikan tea intake on SHRs Data for BW, food intake, BP, and HR of SHRs before and after the 20-week protocol are summarized in Table 1. No significant differences were observed in BW and food intake during the 20-week daily intake of hesperidin and Mikan tea compared to the control group, suggesting that the intake of hesperidin and Mikan tea at a dose of 50 mg/kg did not affect the growth parameters of SHRs. As shown in Fig. 1, intake resulted in a tendency to suppress the promoted SBP of SHRs from 8 to 23 weeks of age. At the end of the protocol (28 weeks of age), a significant decrease in SBP was observed in the hesperidin (ΔSBP: 62 mmHg, P < 0.05) and Mikan (ΔSBP: 61 mmHg, P < 0.01) groups, compared to the control group (Table 1). During the experimental period, no significant reduction in HR was observed in the hesperidin or Mikan groups, suggesting that the involvement of the nervous system (Hayakawa et al., 2002) in the observed SBP reduction could be excluded from the hesperidin and Mikan tea anti-hypertensive mechanism.
| 8-week | 28-week | |||||
|---|---|---|---|---|---|---|
| Control | Mikan | Hesperidin | Control | Mikan | Hesperidin | |
| Body weight (g) | 231±3 | 230±3 | 235±3 | 431±12 | 441±9 | 446±0 |
| Food intake (g/week) | 153±2 | 153±3 | 162±5 | 149±10 | 147±8 | 163±11 |
| SBP (mmHg) | 171±11 | 168±6 | 172±10 | 227±4 | 166±15** | 165±23* |
| DBP (mmHg) | 114±12 | 106±3 | 124±4 | 147±11 | 116±11 | 135±15 |
| HR (beats/min) | 458±9 | 457±4 | 468±13 | 420±13 | 428±26 | 408±33 |
Eight-week-old male SHRs were administered Mikan tea (50 mg/kg) or hesperidin (50 mg/kg) daily for 20 weeks. Values are expressed as the mean ± SEM (n = 4).
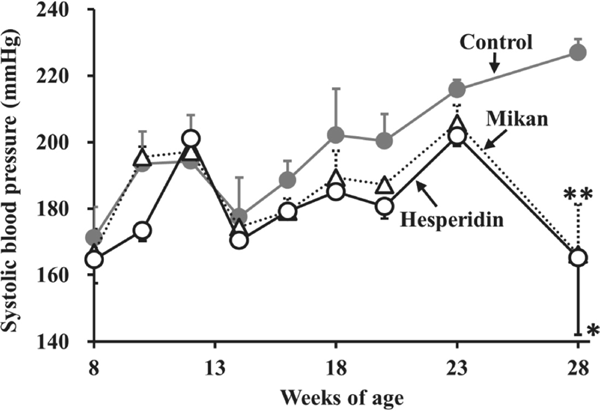
Changes in systolic blood pressure (SBP) of SHRs induced by the intake of Mikan tea, and hesperidin. Eight-week-old male SHRs were administered Mikan tea (50 mg/kg) or hesperidin (50 mg/kg) daily for 20 weeks. Values are expressed as the mean ± SEM (n = 4). *P < 0.05, **P < 0.01 compared to the control by the Student's t-test.
The vasomotor response of the thoracic aorta from hesperidin- and Mikan tea-administered SHRs Maximum contraction/relaxation responses of the aorta against vaso-agonists were examined in 1 mM PE- and subsequently 100 µM ACh-stimulated aortic rings taken from Mikan tea- and hesperidin-administered 28-week-old SHRs (Fig. 2A). As shown in Fig. 2B, no significant difference in the PE-induced contraction response was observed between the groups, whereas ACh-induced relaxation of the aorta in the hesperidin group tended to display a higher response (tension: 0.050 ± 0.005 g, P = 0.19 vs. control), compared to that of the control group (tension: 0.034 ± 0.08 g) (Fig. 2C). Considering the resulting vasomotor profile (Fig. 2D), it appears that the aorta in the hesperidin group exhibited a high vasomotor tone or contraction/relaxation response (P < 0.05) following a 20-week daily intake of hesperidin (likewise, the Mikan group), compared to that of control group.

Effects of Mikan tea and hesperidin daily intake for 20 weeks on the vasomotor response of 28-week-old SHRs thoracic aorta rings. (A) Representative traces were recorded during PE (1 mM)-induced contraction and subsequent ACh (100 µM)-induced relaxation of the aorta rings. Contraction (B) and relaxation (C) tensions were measured for aorta rings taken from each administration group. The ratio of reduced tension by ACh to increased tension by PE was used for the vasomotor response index (D). Values are expressed as the mean ± SEM (n = 4). *P < 0.05 compared to the control by the Student's t-test. N.S., no significance.
Effects of the intake of hesperidin or Mikan tea intake on plasma Ang II, Ang (1–7), NOx, and arterial cAMP in SHRs Levels of circulating RAS-related metabolites, Ang II [a pressor hormone (Qi et al., 2014)], and Ang (1–7) [a depressor hormone (Ferrario, 2010)], as well as that of vasorelaxation-related NOx (Chung et al., 2012), were investigated in 28-week-old SHRs plasma; vasorelaxation factor cAMP levels were similarly evaluated in aorta tissues (Lamping, 2001). After 20 weeks of administration (28 weeks of age), no significant differences in plasma Ang II and Ang (1–7) levels were observed between the hesperidin or Mikan groups and the control group (Table 2), suggesting that their anti-hypertensive effect (Fig. 1) did not involve the suppression of circulating RAS components or the inhibitory action of angiotensin I-converting enzyme (ACE). The absence of plasma NOx level variation in the hesperidin or Mikan group additionally suggests that the anti-hypertensive effect did not involve anti-oxidation or a NOx-led endothelial response. In contrast, although cAMP levels in thoracic aorta of 28-week-old SHRs did not increase significantly in the hesperidin group (P = 0.30), the increasing tendency allows a speculation regarding the involvement of hesperidin in the cAMP/protein kinase A (PKA) vasorelaxation axis (García-Morales et al., 2017).
| Control | Mikan | Hesperidin | |
|---|---|---|---|
| Plasma Ang II (pmol/mL) | 0.043±0.003 | 0.061±0.021 | 0.034±0.003 |
| Plasma Ang (1–7) (pmol/mL) | 1.8±0.1 | 1.7±0.1 | 1.8±0.1 |
| Plasma NOx (nmol/mL) | 17.6±0.5 | 18.7±3.9 | 15.4±0.9 |
| Arterial cAMP (pmol/mg protein) | 91.1±18.4 | 71.0±19.4 | 178.7±58.1 |
Parameters were measured at the end of the 20-week protocol in SHRs for the hesperidin and Mikan tea groups. Values are expressed as the mean ± SEM (n = 4). No significant differences were obtained at P < 0.05 by the Student's t-test.
Effects of hesperidin and Mikan tea on AT1R, AT2R, and MasR expression in SHRs Wes analyses of AT1R, AT2R, and MasR expression in thoracic aorta were performed after the 20-week protocol of hesperidin and Mikan tea intake by SHRs. As shown in Fig. 3A–D, the hesperidin and Mikan groups that exhibited a high vasomotor response (Fig. 2) did not affect the expression of contraction/relaxation-related receptors, AT1R and AT2R, in the aorta, compared to the control group. In contrast, levels of MasR, an up-streamed G-protein-coupled receptor (Povlsen et al., 2020) for the vasodilation factor, were significantly (P < 0.05) increased in the aorta of hesperidin-administered SHRs (hesperidin group: 295.6 ± 40.5% vs. control, similar to the Mikan group: 242.2 ± 31.5% vs. control) (Fig. 3E, F), suggesting that hesperidin exerts its physiological activity via aorta relaxation, arising from MasR/cAMP signaling pathway activation.

Protein expression of AT1R (A, B), AT2R (C, D), and MasR (E, F) in the thoracic aorta of 28-week-old SHRs. Thoracic aortas taken from Mikan tea- or hesperidin-administered SHRs were subjected to Wes analysis. The chemiluminescent signal is displayed as a virtual blot-like image and an eletropherogram was generated based on the molecular weight. Protein expressions of AT1R (B), AT2R (D), and MasR (F) were normalized by the electropherogram peak area of the applied total protein in each lane. Values are expressed as the mean ± SEM (n = 4). Statistical differences between the hesperidin group (or Mikan group) and the control group were evaluated by the Student's t-test. *P < 0.05 compared to the control. N.S., no significance.
In the present study, a 20-week administration of hesperidin (50 mg/kg), or Mikan tea (50 mg/kg), containing 0.56 mg/kg hesperidin to SHRs, resulted in a significant anti-hypertensive effect by SBP reduction (Fig. 1). At 28 weeks of age, the degraded vasomotor tone (i.e., contraction/relaxation response) of SHRs was significantly ameliorated by the intake of hesperidin and Mikan tea (Fig. 2), through the activation of the MasR/cAMP vasorelaxation axis. Our previously reported human study revealed that a 12-week intake of fermented Mikan tea (890 mg/day), rich in hydrophobic hesperidin (36.7 mg/890 mg-Mikan tea) (Nakayama et al., 2014), significantly lowered (approximately 8 mmHg) elevated SBP in 20 high-normal and mildly hypertensive subjects (K. Tanaka et al., 2020). However, studies investigating the mechanism responsible for the anti-hypertensive effects of hesperidin present in Mikan tea have not been conducted, although natural phytochemicals, such as epigallocatechin gallate (EGCG) (Potenza et al., 2007), quercetin (Duarte et al., 2001), and (-)-catechin (Quiñones et al., 2015), have exhibited in vivo BP-lowering effects in SHR experiments. The magnitude of hesperidin-induced SBP reduction (ca. 60 mmHg/50 mg dose) in this SHR study (Table 1) was higher than that of the above-mentioned phytochemicals, taking their dosage into account (e.g., ΔSBP of EGCG, ca. 50 mmHg/200 mg dose (Potenza et al., 2007); ΔSBP of quercetin, ca. 40 mmHg/10 mg dose (Duarte et al., 2001); ΔSBP of (-)-catechin, 29.9 mmHg/0.5 mg dose (Quiñones et al., 2015). This suggests that hesperidin, a candidate in Mikan tea, may exert more potent anti-hypertensive effects among the reported anti-hypertensive phytochemicals. However, by considering the low content of hesperidin in dose of Mikan tea (0.56 mg/kg in 50 mg-Mikan tea/kg), the involvement of other compounds in the anti-hypertensive effect of Mikan tea (Table 1) cannot be ruled out, and further experiments are needed to clarify the candidates responsible for the effect in terms of their synergic and combinatorial action.
Considering that the RAS plays a pivotal role in BP regulation (Yang and Xu, 2017), and that vessel resistance determines BP elevation (Michael et al., 2008), the effects of hesperidin on typical BP-regulatory Ang metabolites and vasomotor factors were examined in the plasma and thoracic aorta of 28-week-old SHRs. It was evident that hesperidin intake did not affect levels of the plasma pressor, Ang II, as an index of ACE activity (Brosnihan et al., 1999), nor the level of the plasma depressor, Ang (1–7), as an index of ACE2 activity (Ferreira and Raizada, 2008), during the 20-week protocol (Table 2), suggesting that the circulating RAS components are not involved in the hesperidin-induced anti-hypertensive effect. No changes in plasma NOx levels were observed in hesperidin-administered SHRs (Table 2), indicating that the antioxidant action against oxidative stress in the vascular endothelium could be excluded from hesperidin effects. In contrast to the present findings, previous animal studies using hypertensive rats have purposed that the anti-hypertensive mechanism of hesperidin in 2K-1C hypertensive Sprague-Dawley (SD) rats (Wunpathe et al., 2018) or glycosylated hesperidin in SHRs (Yamamoto et al., 2008) was due to RAS downregulation and/or endothelial oxidative stress-related cascades. The contradiction between the presented anti-hypertensive mechanism and those of the above-mentioned reports could be due to the difference in rat species or the use of glycosylated hesperidin. Further studies are currently ongoing to clarify the underlying anti-hypertensive mechanisms of hesperidin activity, using vascular endothelial cells. Reportedly, the effects of glucuronized hesperetin, a possible hesperidin metabolite, on BP reduction, differs from that of its conjugated metabolites in SHRs (Yamamoto et al., 2013); this allows for another speculation that the anti-hypertensive action of hesperidin may be influenced by its resultant metabolites. In our previous report (Nectoux et al., 2019), we demonstrated that oral administration of hesperidin (10 mg/kg) to SD rats resulted in the production of a variety of metabolic conjugates, such as homoeriodictyol and eriodictyol, in their sulfated and glucuronized forms, along with the higher absorption of 6.4 nmol·h/mL-plasma rather than those of theasinensins (approximately 20 pmol·h/mL-plasma) (Matsui, 2015).
In the present study, we established that the intake of hesperidin resulted in a significant enhancement of MasR protein expression in the vascular tissues of SHRs, while vasomotor-related receptors, AT1R and AT2R, were not affected (Fig. 4). To the best of our knowledge, this is the first report wherein the observed hesperidin-induced anti-hypertensive effect was found to involve the upregulation of MasR protein expression in vascular tissues. MasR can recognize the depressor Ang (1–7), which plays a role in hypertension by counterbalancing vasoconstriction by promoting the cAMP vasorelaxation cascade (Bader et al., 2018). Reportedly, the MasR axis is upregulated by p38 mitogen-activated protein kinase (MAPK) inhibition (Chen et al., 2013), and hesperidin has been identified as a p38 MAPK inhibitor in in silico studies (Selim et al., 2019). Considering that the plasma Ang (1–7) levels did not increase by hesperidin intake (Table 2), we can assert that hesperidin potentially plays a role in the upregulation of MasR expression via p38 MAPK inhibition, but not via an Ang (1–7) production caused by ACE2 activation (Fig. 4). However, the precise mechanisms require further investigation.

Hesperidin-induced vasorelaxation in SHR aorta.
The present study demonstrates that long-term administration of hesperidin and Mikan tea, each at a dose of 50 mg/kg/day, for 20 weeks to 8-week-old SHRs, resulted in a significant BP-lowering effect, together with a potent vasomotor response in the thoracic aorta. MasR expression in the aorta was significantly upregulated by hesperidin, while factors related to the circulating and local RAS were not influenced. Taken together, these results suggest that hesperidin plays a crucial role in the stimulation of the MasR/cAMP vasorelaxation axis in SHRs, leading to an anti-hypertensive effect.
The authors declare no conflicts of interest.