2020 年 26 巻 6 号 p. 837-846
2020 年 26 巻 6 号 p. 837-846
Citrus natsudaidai is a popular citrus in Japan, but the peel is of limited use and its composition is not well documented. The purposes of this study were to obtain health-beneficial compounds from C. natsudaidai peel for food use by simple methods and to investigate the antioxidant capacities of extracts of different polarities without overestimating the effects. C. natsudaidai peel was extracted with water-ethanol and hexane-ethanol solutions to obtain five different soluble fractions. The highest total phenolic content (TPC) was obtained from the water-soluble fraction (WSF), and the lowest content was observed in the hexane soluble fraction (HSF). The WSF also showed the highest antioxidant activities, and the lowest activities were found in HSF. A positive correlation was detected between TPC and antioxidant activities. The polar extract of C. natsudaidai peel has potential as a natural additive or ingredient in foods, cosmetics and pharmaceutical products with antioxidant activity.
Plant-based foods and their processing wastes such as the peel, seeds, and leaves are well known to contain several antioxidants that may positively affect human health (Takebayashi et al., 2013). Citrus species are one of the main sources of vitamin C, flavonoids, carotenoids, and other phenolic compounds which have high antioxidant activity (Zhang et al., 2014; de Moraes Barros et al., 2012; Ramful et al., 2010). In most cases, the peel contains higher amounts of these compounds than the pulp (Assefa et al., 2017; de Moraes Barros et al., 2012). However, huge quantities of citrus peel waste are generated all over the world during processing and are discarded. Citrus natsudaidai is a particularly popular citrus fruit in Japan, and it has a singular characteristic in that it has a very thick peel that is hard to remove. It is difficult to manage effectively, and only a few studies have focused on C. natsudaidai peel. Alternative means of valuation should be considered to contribute to waste reduction. Our previous report (Matsuo et al., 2019) showed that C. natsudaidai peel extract includes beneficial compounds that can be used as natural additives for foods or cosmetics. Therefore, the next step of this work looks at evaluating health-promoting effects, such as antioxidant capacity.
Compounds present in plant-based foods and their processing wastes have different chemical features, such as molecular weight, arrangement of branching, linkage type and degree, which can influence their physicochemical behavior (Zhang and Tsao, 2016). Considering the extraction conditions, polarity and solubility are important since these features have a huge effect on extract yield and composition. For instance, hydrophilic compounds can be extracted with polar solvents such as water, ethanol, and methanol, whereas chloroform, ether, and hexane are commonly used for the extraction of hydrophobic compounds (Azmir et al., 2013).
To obtain compounds present in plant-based foods and their processing wastes, conventional solvent extraction is usually performed since it can be conducted at room temperature and at atmospheric pressure. Many researchers have shown interest in extraction methods of citrus peel. M'hiri et al. (2015) compared various extraction techniques (conventional solvent extraction, microwave-assisted extraction, ultrasound-assisted extraction, high hydrostatic pressure extraction, and supercritical fluid extraction) to investigate the total phenolic content (TPC) and the antioxidant activity of Citrus sinensis peel. Chan et al. (2009) studied the effect of extraction condition (solvent type, concentration, temperature, and time) on the TPC from Citrus hystrix and determined the optimum conditions to be 52.9% aqueous ethanol, 48.3°C, and 126.3 min of extraction time. Other reports compared the impact of various extraction solvents on citrus peel TPC and antioxidant activity using 2,2-diphenyl-1-pikrylhydrazyl (DPPH) radical. Lou et al. (2014) found the highest TPC and DPPH radical scavenging activity in water extract compared to ethanol, methanol, and ethyl acetate extracts. Park et al. (2014) found the highest TPC in acetone extract followed by methanol and ethanol extract, while acetone showed the lowest DPPH radical scavenging activity, and methanol revealed the highest activity. Hegazy and Ibrahium (2012) compared six extraction solvents: methanol, ethanol, dichloromethane, acetone, hexane, and ethyl acetate. TPC was higher in the order of ethanol > methanol > acetone > dichloromethane > ethyl acetate > hexane, and DPPH radical scavenging activity followed the order ethanol > methanol > ethyl acetate > acetone > dichloromethane > hexane.
Most of the studies that investigated different solvents for citrus peel extraction are focused on obtaining the best yield of phenolic compounds and antioxidant activities (Lou et al., 2014; Park et al., 2014; Hegazy and Ibrahium, 2012), regardless of the presence of overlaying components in extracts with similar polarity. For instance, the most commonly used solvents seem to be ethanol, methanol, and acetone, which are polar to intermediate polar solvents. Thus, some compounds that are soluble in ethanol could also be soluble in methanol and acetone, and therefore TPC or the antioxidant activity can be estimated considering overlapping compounds and not compounds specifically extracted by only one solvent type. We propose here an original extraction procedure that avoids overlapping extracted components to determine the TPC combined with the estimation of antioxidant activity of C. natsudaidai peel based on DPPH and 2,2-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical scavenging activity.
To our knowledge, no study has been conducted on the antioxidant activity of C. natsudaidai peel extracts prepared with different polar solvents avoiding overlapping composition. Therefore, the objectives of this study were set to (1) obtain extracts of C. natsudaidai peel with food-use solvents and cost-effective methods; (2) investigate the TPC and antioxidant properties without overestimating; (3) compare the TPC and antioxidant activities of each extract of different polarity.
Chemicals Ethanol, hexane, and sodium sulfate were purchased from Kanto Chemical Co., Inc. (Tokyo, Japan). Milli-Q water was prepared by Milli-Q® Integral A10® System (Molsheim, France). Sodium carbonate, potassium persulfate, and ABTS were purchased from Wako Pure Chemicals Industries, Ltd. (Osaka, Japan). DPPH, 6-Hydroxy-2,5,7,8-tetramethyl-chromane-2-carboxylic acid (Trolox), and Folin-Ciocalteu phenol reagent were acquired from Sigma-Aldrich Co. (St. Louis, MO, USA). Gallic acid hydrate was purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan).
Samples Three different cultivars of C. natsudaidai were obtained from Chiba Prefecture, Japan, namely CN1, CN2, and CN3 in this study. CN1 cultivar is newly developed by a farmer; and CN2 and CN3 are commercial C. natsudaidai fruits from different farmers. All of the fruits were cultivated for 3 years without any use of pesticides and annually harvested from May to July. Upon arrival at Toyo University Food Science Laboratory, the fruits were immediately washed and manually peeled. Approximately 500 g of peels per cultivar were crushed using a hand blender (Click & Mix Plus T-fal, Tokyo, Japan) and stored at −30°C before any other treatment.
Conventional solvent extraction Two solvent mixtures (water-ethanol and ethanol-hexane) were used as extraction solvents. From the perspective of yield and efficiency, the ethanol ratio of water-ethanol mixture and extraction time were set at 80% (v/v) and 24 h, respectively (Lou et al., 2014; Chan et al., 2009; Li et al., 2006). Ethanol-hexane mixture was prepared in a 2:1 (v/v) ratio (Bligh and Dyer, 1959), and extraction time was also set at 24 h. To prevent the thermal degradation of antioxidants, extraction was carried out at room temperature (25°C).
Extraction with water-ethanol mixture Four polar extracts were obtained following the method described by Zhang et al. (2011) and Li et al. (2006) with some modifications. Ground frozen citrus peel (5 g) was extracted with 80% aqueous ethanol (v/v) with a 1:10 (w/v) ratio of sample:solvent under magnetic stirring for 24 h at room temperature (25°C). A first extract, namely non-defatted ethanol extract (NDEE), was obtained by centrifuging the extract at 8 000 rpm (7 090 × g) for 5 min at 4°C. Then, the supernatant was collected in a round-bottomed flask through filtration with Advantec® 5B 150 mm filter paper. Residues were re-extracted twice by hand-shaking (1 min) following the same procedure. Further, the filtrated supernatant was evaporated in a rotary evaporator to dryness, and then dissolved in 100% ethanol. The extract was then transferred to a volumetric flask and filled up to 25 mL. The extract was filtered on with a hydrophilic nylon 0.22 µm syringe filter. A second extract, named defatted ethanol extract (DEE), was obtained similarly as shown in Figure 1, except that the extract was evaporated until the volume became less than 5 mL. The remaining extract was then quantitatively transferred in a separatory funnel by the addition of Milli-Q water, ethanol and hexane (3 times, 10 mL of each solvent) before being submitted to liquid-liquid partition. After clear separation, the hydrophilic phase was transferred to a second separatory funnel and was defatted with 20 mL of hexane another two times. The hydrophilic phase was concentrated in a second round-bottomed flask, followed by evaporation until dryness, dissolved in 100% ethanol and then, transferred to a 25 mL volumetric flask. At last, filtration was performed. A third extract, namely the WE, was obtained following similar conditions as the DEE. The second evaporation step was ended when the remaining extract became less than 5 mL, and the remaining extract was dissolved with Milli-Q water and transferred to a 25 mL volumetric flask, followed by filtration. After this procedure, a water-insoluble residue (as shown in Figure 2) was formed on the wall of the round-bottomed flask. To collect the remaining part of the extract, the flask of WE was subjected again to evaporation to dryness and then the residue was dissolved in 100% ethanol in a 10 mL volumetric flask. The extract was filtered and was called defatted+dehydrated ethanol extract (DDEE). All the extracts were stored at −30°C until further analysis.
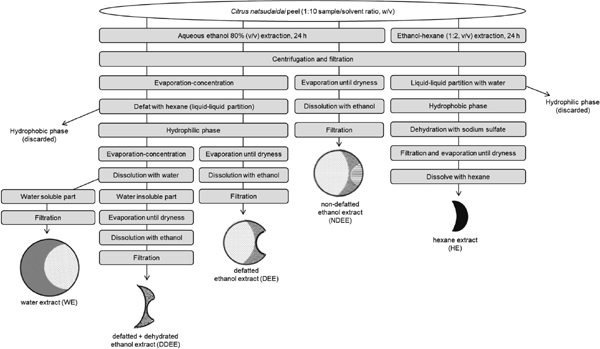
The extraction method used to obtain five different extracts.

Representation of the five extracts obtained from the extraction method (Figure 1), showing that they have overlapping soluble parts (A); Illustration of the three main soluble fractions. WE: water extract; NDEE: non-defatted ethanol extract (B).
Extraction with ethanol-hexane mixture The fifth extract, namely hexane extract (HE), was obtained based on the methods of Cheong et al. (2012) and Bligh and Dyer (1959). Ground frozen citrus peels (5 g) were extracted with ethanol-hexane solvent mixture (2:1, v/v) at sample:solvent ratio of 1:10 (w/v) under magnetic twist for 24 h at room temperature. Further, the sample was centrifuged at 8 000 rpm (7 090 × g), for 5 min at 4°C, and the supernatant was transferred to a separatory funnel after filtration. Residues were re-extracted with 50 mL of ethanol-hexane mixture by hand-shaking (1 min) twice following the same procedure. All the re-extraction solvents were pooled with the extracted supernatant in the separatory funnel, and 30 mL of Milli-Q water was added. After clear separation, the hydrophilic phase was discarded, and the extract was partitioned again with 30 mL of Milli-Q water another two times following the same procedure. The hydrophobic phase was collected in a conical flask in which sodium sulfate was added to withdraw any remaining water content. The extract was then filtrated to a round-bottomed flask, and evaporated to dryness using a rotary evaporator. It was solubilized with 10 mL of hexane and transferred in a glass tube. The extract was then evaporated to dryness using a tube evaporator and stored at −30°C until further analysis.
Determination of TPC The TPC of citrus peel extracts was measured using the Folin-Ciocalteu method described by Sheikh et al. (2009) with minor modifications. Briefly, 0.5 mL aliquot of sample or of gallic acid standard was placed in a glass tube, mixed with 0.75 mL Milli-Q water and 0.25 mL of Folin-Ciocalteu reagent. Samples were diluted according to their solvent solution (Milli-Q water, ethanol or hexane). After 1 min, 0.5 mL of saturated sodium carbonate solution was added and mixed again. Then, it was allowed to react for 90 min at room temperature, and the absorbance was measured at 765 nm using a UV-VIS spectrophotometer (UV-1800, Shimadzu Corporation, Kyoto, Japan). A blank was prepared using Milli-Q water instead of the sample solution. Results were expressed as mg of gallic acid equivalent (GAE) per 100 g of citrus peel.
ABTS radical scavenging activity The ABTS radical scavenging activity of citrus peel extracts was determined according to the method of Marathe et al. (2011) with some modifications. Briefly, 7 mM of ABTS was prepared with Milli-Q water and potassium persulfate was added with mixing to a 2.45 mM concentration. This solution was left overnight at 4°C with protection from light exposure. Before analysis, the ABTS stock solution was diluted with ethanol at a ratio of 1:89 (v/v). The reaction mixture consisted of 0.8 mL of ABTS solution and 0.2 mL of citrus peel extract at different concentrations, which were diluted with ethanol. The mixture was shaken and left to react for 30 min at room temperature in the dark. The absorbance at 734 nm was recorded by a UV-VIS spectrophotometer. Ethanol was used as the blank. A control was prepared using ethanol instead of the sample solution. Half maximal inhibitory concentration (IC50) values, which correspond to the concentrations required to reach 50% of the antioxidant effect, were obtained by calculation. Trolox was used as the reference antioxidant compound, and the results were expressed as mg of trolox equivalent (TE) per 100 g of citrus peel.
DPPH radical scavenging activity The DPPH radical scavenging activity of citrus peel extracts was determined using a modified method based on Sheikh et al. (2009). Briefly, 0.5 mL of 0.1 mM DPPH ethanolic solution was mixed with 0.5 mL of citrus peel extract at different concentrations diluted with ethanol. The mixture was shaken and left to stand in the dark for 30 min at room temperature. Absorbance was measured at 517 nm using a UV-VIS spectrophotometer. Ethanol was used as the blank. The control consisted of the DPPH solution and ethanol. DPPH radical scavenging activity was obtained by the same calculation as for the ABTS assay.
Statistical analysis All experiments were performed in triplicate and the results were expressed as the mean ± standard deviation (SD). The data were analyzed using the Statistical Package for Social Sciences (IBM® SPSS® Statistics for Windows version 22). Statistical significance was declared at p < 0.05.
As shown in Figures 1 and 2, five extracts were obtained using two solvent mixtures: water/ethanol and ethanol/hexane. Ethanol has an acceptable cost and is safe as it is categorized as generally recognized as safe (GRAS) (FDA, 21 CFR 184-Substances Affirmed as GRAS in Food as of 04 October 2019). Hexane is permissible for extracting fats or oils in the manufacturing of edible fats or oils in Japanese industry (MHLW, Standards for Use of Food Additives of 06 June 2019). Moreover, thermal processing and additional equipment(s) to control temperature and pressure were not necessary. Therefore, the extraction method used in this study is useable in foods and is an environmentally friendly and cost-effective process.
Water solubilizes hydrophilic compounds regardless of molecular weight. Since ethanol is an intermediate-polar solvent, it can extract hydrophilic to hydrophobic compounds. However, high molecular weight compounds such as polysaccharides and proteins are not solubilized. Moreover, ethanol can be well mixed with water and hexane. Hexane can extract hydrophobic compounds such as fat; therefore, it was also used to defat the aqueous solution.
Extracted compounds that are water-soluble can be mixed with ones that are soluble in ethanol and, vice-versa, thus creating an overlapping part, as shown in Figure 2. The extracts obtained in this study contain overlapping parts. However, through calculations, it was possible to estimate the TPC and the total antioxidant activity without considering overlapping compounds in the results. To eliminate the overlapping parts, TPC and the antioxidant activity of C. natsudaidai peel extract were calculated and divided into five soluble parts (Figure 2): water-only soluble, water+ethanol mix soluble, ethanol-only soluble, ethanol+hexane mix soluble, and hexane-only soluble part. These were called “soluble parts” to distinguish them from extracts. Further, these data were combined to calculate the TPC and antioxidant activity of the three different soluble fractions, water-soluble fraction (WSF), ethanol-soluble fraction (ESF) and hexane-soluble fraction (HSF), as shown in Figure 2, and they were named the “soluble fraction”.
The WE fraction was extracted using 80% ethanol as the solvent (Figure 1). Therefore, it is reasonable to conclude that water soluble and ethanol soluble compounds were obtained in this process. This includes the water-only soluble, water+ethanol mix soluble, ethanol-only soluble, and ethanol+hexane mix soluble parts, which are represented in Figure 2. Then, continuing the extraction process, the extract was defatted with hexane; hence, the compounds that were in the ethanol+hexane mix soluble part were removed. After all solvents were evaporated (80% ethanol and hexane), water was added and water-soluble compounds (water-only soluble and water+ethanol mix soluble parts) were solubilized (named WE). Water-insoluble compounds (only soluble in ethanol) remained in the flask. Ethanol was added after drying the flask, and the remaining compounds were solubilized in ethanol (named defatted+dehydrated ethanol extract, DDEE). For defatted ethanol extract (DEE), the same process as for WE was conducted until the defatting step (using hexane). After evaporation, water-only soluble, water+ethanol mix soluble, and ethanol-only soluble parts were all present in the flask. Ethanol was added, dissolving the ethanol soluble compounds (water+ethanol mix soluble and ethanol-only soluble parts). Non-defatted ethanol extract (NDEE) was obtained using the same process as for DEE but without the defatting step. Thus, NDEE includes the ethanol+hexane mix soluble part. The HE differed from the others in that ethanol-hexane mixture was used for extraction; hence, ethanol soluble and hexane soluble compounds (water+ethanol mix soluble, ethanol-only, ethanol+hexane mix soluble, and hexane-only soluble parts) were extracted first. Water was added and the hydrophilic phase was discarded (water+ethanol mix soluble, ethanol-only soluble, and ethanol+hexane mix soluble parts). The “hydrophilic phase” includes water soluble and ethanol soluble compounds. Since ethanol will mix with water more easily than hexane, ethanol was removed with water. Finally, the hexane-only soluble part remained in the flask, and it was solubilized with hexane.
Determination of TPC TPC of C. natsudaidai peel extracts, soluble parts, and soluble fractions is summarized in Table 1. Concerning the extracts (WE, DDEE, DEE, NDEE and HE), WE had the significantly highest TPC (320–369 mg GAE/100 g FM) followed by NDEE (149–185 mg GAE/100 g FM) and DEE (116–128 mg GAE/100 g FM). For the soluble parts (water-only, water+ethanol mix, ethanol-only, ethanol+hexane mix and hexane-only), the water-only soluble parts exhibited the highest TPC (204–242 mg GAE/100 g FM), followed by the water+ethanol mix soluble parts (115–127 mg GAE/100 g FM) and the ethanol+hexane mix soluble parts (20.8–68.6 mg GAE/100 g FM). Regarding the soluble fractions (WSF, ESF, and HSF), the WSF(= WE) showed the highest TPC followed by the ESF(= NDEE) and the HSF for all cultivars.

From the comparison of TPC in C. natsudaidai cultivars, CN1 and CN3 showed higher TPC in the WSF(= WE) than did CN2. While present in ESF(= NDEE) and HSF, CN2 had significantly higher TPC than CN1 and CN3. The total amount of phenolics (sum of all “soluble parts”) showed no significant differences.
ABTS radical scavenging activity The ABTS radical scavenging activity of C. natsudaidai peel extract is reported in Table 2. The WE exhibited the highest antioxidant activity (132–159 mg TE/100 g FM) followed by the NDEE (51.7– 56.5 mg TE/100 g FM) and the DEE (32.0–43.9 mg TE/100 g FM) for all cultivars. After removing the overlapping part in the same way as TPC (Table 1), the water-only soluble part represented significantly the highest value (100–117 mg TE/100 g FM), followed by the water+ethanol mix soluble part (31.6–42.7 mg TE/100 g FM) and the ethanol+hexane mix soluble part (9.92–21.8 mg TE/100 g FM) in all of the three cultivars. The WSF(= WE) displayed significantly the highest antioxidant activity followed by the ESF(= NDEE) and the HSF.

When comparing the antioxidant activity among the cultivars, CN1 and CN3 showed significantly higher values than CN2 in the WSF(= WE), whereas there were no significant differences in the ESF(= NDEE) and the HSF. Besides, total value (sum of each of all “soluble parts”) showed no significant difference. In this study, the ABTS value of C. natsudaidai extracts was higher in the water (polar) part, and lower in the hexane part.
DPPH radical scavenging activity Table 3 shows the DPPH radical scavenging activity of C. natsudaidai peel extracts. The WE exhibited the highest DPPH value (34.2–37.8 mg TE/100 g FM) followed by the NDEE (30.2–33.0 mg TE/100 g FM) and the DEE (20.7–29.7 mg TE/100 g FM) for all cultivars. Considering the soluble parts, the water+ethanol mix soluble part clearly displayed the highest activity (20.6–29.1 mg TE/100 g FM) followed by the water-only soluble part (8.79–13.6 mg TE/100 g FM), and the ethanol-hexane mix soluble part (3.32–10.9 mg TE/100 g FM). Among the three cultivars, there were no significant differences in the WSF and the ESF, as well as for the total DPPH values (each of the “soluble parts” sums). From all the results, it was observed that the DPPH radical scavenging activity of C. natsudaidai peel extracts is higher in the polar and intermediate-polar parts, and lower in the non-polar parts.

Correlation between the TPC and the antioxidant activities Pearson's correlation coefficients were calculated to determine the relationship between the TPC and the antioxidant activities (Figure 3). There were strong positive correlations among them (TPC × ABTS: r = 0.9851; TPC × DPPH: r = 0.8985; ABTS × DPPH: r = 0.8309).

Correlation of total phenolic content and antioxidant activities of Citrus natsudaidai peel extracts. Pearson R: Pearson's correlation coefficient; TPC: total phenolic content; ABTS: ABTS radical scavenging activity; DPPH: DPPH radical scavenging activity; mg GAE/100 g FM: mg of gallic acid equivalent per 100 g of sample based on fresh matter weight; mg TE/100 g FM: mg of Trolox equivalent of sample based on fresh matter weight. All correlations were significant at p < 0.01.
The results of the TPC determination clarified that the phenolic compounds in C. natsudaidai peel are present mainly in the polar part. The TPC of citrus peel extract varies among citrus species. After adjusting the units to compare with our study, Ramful et al. (2010) found a TPC ranging from 188.2 to 766.7 mg GAE/100 g FM in citrus peel 80% methanol extracts from 36 varieties. Lagha-Benamrouche and Madani (2013) showed that 7 kinds of citrus peel extracts, obtained with 80% methanol contained from 241 to 765 mg GAE/100 g FM. These reports did not include the use of other extraction solvents. Comparing the TPC between different polar solvents, C. natsudaidai peel showed that the WSF(= WE) includes 1.7 to 2.5 times higher amounts than the ESF(= NDEE) and 4.7 to 16 times higher amounts than HSF. The ESF(= NDEE) comprised a 2.6 to 6.7 times higher TPC than the HSF. Some other previous reports showed a similar pattern. Hegazy and Ibrahium (2012) described that ethanol extract of the dried orange peel powder contained 2.7 times higher TPC than the HE. Emam and El-bassyouni (2015) also found that the TPC of dried orange peel ethanol extract had 1.3 times higher TPC than its HE. Although the amounts of phenolics vary, those results revealed that phenolic components in citrus peel distribute mainly on the polar to intermediate-polar parts.
Regarding antioxidant properties, Rekha and Bhaskar (2013) determined the DPPH radical scavenging activity of orange peel extract prepared with several solvents including ethanol and hexane. They reported that the hexane extract showed higher antioxidant activity than the ethanol extract. Hegazy and Ibrahium (2012) and Lou et al., (2014) also reported DPPH radical scavenging activity of the citrus peel extracted with different solvents. The authors found a similar trend to our results: DPPH radical scavenging activity is higher in the polar extracts of citrus peel. Jayaprakasha et al. (2008) reported the ABTS radical scavenging activity in various extracts of pomelo and navel orange “edible parts” and showed stronger activity of ABTS inhibition in the methanol extract and a lower activity in the hexane extract. Sahreen et al. (2010) reported ABTS and DPPH radical scavenging activity of Carissa opaca fruit in various extracts. The authors indicated that the best antioxidant values of the extracts were aqueous > methanol > hexane for both radicals. Barbouchi et al. (2020) described the DPPH radical scavenging activity of Pistacia lentiscus twigs, leaves, and fruit extracted with different solvents, and they showed that the best antioxidant values of the fruit extract were aqueous > ethanol > hexane extract. These findings are consistent with the results presented here.
Several previous studies reported that the TPC of citrus peel showed positive correlation with their antioxidant activity (Lou et al., 2014; Lagha-Benamrouche and Madani, 2013; Ramful et al., 2010). In contrast, de Moraes Barros et al. (2012) reported no correlation between the TPC and the antioxidant activity in citrus peel extract. Rahman et al. (2018) founded a negative correlation between TPC and DPPH values. The differences in the studied citrus species and the extraction procedure could explain this diversity. Citrus peel contains several phenolic compounds such as phenolic acids and flavonoids, and their composition varies among species (Lou et al., 2014; Zhang et al., 2011; Ramful et al., 2010). The majority of phenolic compounds act as good antioxidants, while some compounds have weak activity (Pannala et al., 2001). Besides, the presence of other bioactive compounds such as vitamins, terpenoids, mineral elements, amino acids, fatty acids, and pectin probably could affect antioxidant capacities (Matsuo et al., 2019; Assefa et al., 2017; Zou et al., 2016; de Moraes Barros et al., 2012).
Extractions using solvents of various polarities compatible with the food industry process were conducted on C. natsudaidai peel to obtain antioxidant compounds. Then, the TPC and the antioxidant properties were determined. The TPC and the antioxidant properties were higher in the polar extracts, and there was a high correlation between the TPC and antioxidant activity. Polar extracts of C. natsudaidai peel with antioxidant activity have the potential to be used as a natural food additive or ingredients for cosmetic and pharmaceutical products. From this perspective, it would be of great interest to obtain information on the identification of phenolic compounds present in polar extracts and to investigate their respective antioxidant activities.