2020 年 26 巻 6 号 p. 847-854
2020 年 26 巻 6 号 p. 847-854
The number of patients suffering from type I hypersensitivity, including hay fever and food allergies, is increasing. Recent studies have focused on food factors with fewer side effects. Previous studies have suggested that polysaccharides from seaweed suppress type I hypersensitivity by intraperitoneal administration. The aim of this study was to evaluate the inhibitory effect of polysaccharides extracted from dietary Pyropia yezoensis f. narawaensis (PPY) on type I hypersensitivity. Oral administration of PPY inhibited ear edema in the passive cutaneous anaphylaxis (PCA) reaction, but failed to have any effect when injected intraperitoneally. Oral administration of PPY for 4 days significantly increased interleukin (IL)-10 levels in serum as compared with the control group. Moreover, the inhibitory effect of PPY on ear edema was cancelled out by intravenous injection of anti-IL-10 antibody. On the other hand, it was demonstrated that PPY did not suppress β-hexosaminidase release in either a RBL-2H3 cell mono-culture or a Caco-2/RBL2H3 co-culture system, indicating that PPY does not inhibit RBL-2H3 degranulation either directly or through intestinal epithelial cells. In conclusion, dietary PPY could exert anti-allergic effects through the induction of IL-10 secretion in blood, suggesting that red algae may be effective in ameliorating type I hypersensitivity.
Allergic diseases are caused by excessive immune reactions against allergens, and they have become a serious global public health concern (Galli et al., 2008). Allergy, also called hypersensitivity, is divided into four major types, which are designated type I to IV based on the pathogenic mechanism involved (Madore and Laprise, 2010). In addition to these four types, type V has also been suggested as granulomatous hypersensitivity (Rajan, 2003). Type I hypersensitivity, often called immediate-type hypersensitivity, is caused by immunoglobulin E (IgE)-mediated reactions, and it includes diseases such as food allergies, rhinitis, and asthma. The number of patients with type I hypersensitivity has continued to increase worldwide over time (Galli et al., 2008).
Type I hypersensitivity is initiated by allergens, including food factors, pollen, and dust. When an allergen gains access to the human body, it is recognized by antigen-presenting cells (APC) and is presented to naïve helper T cells (Th0). Th0 are activated and differentiate into T helper 2 cells (Th2) rather than T helper 1 cells (Th1). Th2 are capable of secreting Th2 cytokines, including interleukin-4 (IL-4), IL-5, and IL-13, which can promote B cells to undergo immunoglobulin (Ig) class switch recombination, leading to the accumulation of IgE. When the same allergen invades again, the binding of the allergen to IgE activates the Fcε Receptor I (FcεR I) on mast cells, and causes mast cell degranulation, resulting in the release of chemical mediators such as histamine (Janeway Jr. et al., 2001). Therefore, alterations in the Th1/Th2 balance may be important for improving allergic responses. Some patients are concerned about the adverse effects of current conventional drugs for managing allergic diseases, and seek alternative treatments. Cell and animal experiments focused on nutritional adjunctive therapy, an alternative treatment, have shown that vitamins A, E, and C, folate, and fish oil can relieve allergy symptoms (Han et al., 2015).
Seaweed is a source of novel bioactive compounds, such as polysaccharides and phlorotannins, that may confer certain health-promoting properties. Therefore, seaweed has great potential as a food factor with a beneficial function. It has been reported that polysaccharides from red algae improve skin conditions (Doi et al., 2017), have an anti-inflammatory effect against colitis (Sudirman et al., 2018), and suppress the production of the pro-inflammatory cytokine tumor necrosis factor (TNF)-α with the induction of inducible-nitric oxide synthase (Isaka et al., 2015). In addition, it has been reported that polysaccharides and a chromoprotein from red algae suppress type I hypersensitivity by intraperitoneal administration (Liu et al., 2015). Other studies have indicated that fucoidan, a polysaccharide from brown algae, can improve allergic responses by regulating the immune response through alterations in the Th1/Th2 balance, inhibiting the production of IgE, suppressing the degranulation of mast cells, and inhibiting the proliferation of airway smooth muscle (Liu et al., 2016). Tanino et al. (2016) reported that the oral administration of fucoidan prepared from Saccharina japonica inhibited type I hypersensitivity by inducing secretion of galectin-9 into the blood. Moreover, it was demonstrated that its oral administration caused anti-allergic effects after allergen sensitization (Mizuno et al., 2020).
Pyropia yezoensis f. narawaensis, a kind of red alga, is generally cultured in Japan. However, the type I hypersensitivity-suppressing effect of the oral administration of P. yezoensis f. narawaensis has not yet been reported. In this study, the anti-allergy activity of polysaccharides from P. yezoensis f. narawaensis (PPY) was evaluated using a passive cutaneous anaphylaxis (PCA) reaction, and the inhibitory effects of PPY on IgE-mediated allergic responses were further investigated using a co-culture system composed of Caco-2 and RBL-2H3 cells.
Reagents Dulbecco's Modified Eagle's Medium (DMEM, High Glucose) with glutamine was purchased from Wako Pure Chemical Industries (Osaka, Japan). RPMI 1640 medium, trypsin, and MEM non-essential amino acids were purchased from Gibco BRL (Grand Island, NY, USA). Anti-dinitrophenyl (DNP) IgE, p-nitrophenyl N-acetyl-β-D-glucosaminide, and DNP-bovine serum albumin (BSA) were purchased from Sigma (St. Louis, MO, USA). Fetal bovine serum was purchased from Biological Industries (Beit, Israel). 2,4,6-Trinitrochlorobenzene was purchased from Tokyo Chemical Industry (Tokyo, Japan). Mouse anti-2, 4, 6-trinitrophenyl (TNP) IgE was purchased from BD Biosciences (Franklin Lakes, NJ, USA). IL-10 monoclonal antibody, mouse irrelevant IgG, and the IL-10 mouse ELISA Kit were purchased from Life Technologies (Carlsbad, CA, USA).
Mice Female 4-week-old BALB/c mice were purchased from Japan SLC (Shizuoka, Japan). The mice were housed in an air-conditioned animal room at 23 °C ± 2 °C and allowed to acclimate for 7 days before experiments. The mice were maintained in filter-top cages in specific pathogen-free conditions in the Kobe University Life-Science Laboratory with free access to laboratory chow and water ad libitum. All animal experiments were approved and carried out in accordance with the Animal Experiment Ethnics Committee of Kobe University (registration number: 30-10-05).
Separation of each fraction from P. yezoensis f. narawaensis Frozen P. yezoensis f. narawaensis was lyophilized and powdered using a Multi-beads Shocker (Yasui, Osaka, Japan). A powered sample (1 g) was suspended in 25 mL of distilled water, and stirred overnight at room temperature. After centrifugation at 2 330 × g for 15 min, the supernatant was collected and lyophilized, and is referred to as the color fraction from P. yezoensis f. narawaensis (CPY). To remove the remaining chromoprotein, the precipitate from the centrifugation was re-suspended in the same amount of distilled water, and stirred for 1 h at room temperature. The sample solution was centrifuged at 2 330 × g for 15 min, and then the precipitates were dried and incubated overnight at 50 °C with a 50-fold volume of 0.2% acetic acid added to its weight. The sample solution was centrifuged at 2 330 × g for 15 min, and the supernatant was collected. The supernatant was neutralized with 1 M NaOH to pH 7.0, a double volume of 100% ethyl alcohol was added, and the mixture was stirred overnight at 4 °C. Afterward, it was centrifuged at 2 330 × g for 15 min. The precipitate was collected and washed twice with 100% ethyl alcohol. After drying the precipitate, it was dissolved with distilled water, and dialyzed to distilled water overnight to remove salt. The dialysate was lyophilized, and is referred to as the PPY.
Passive cutaneous anaphylaxis (PCA) reaction in mice The IgE-dependent PCA reaction was performed as described previously (Tanino et al., 2016). Briefly, test samples were administered orally for 4 days. After 24 h, mice were sensitized by intravenous injection of anti-TNP IgE antibody. After 30 min, the ear thickness was measured using a thickness micrometer (Peacock G-1A, OZAKI MFG. Co. Ltd., Japan) at baseline, and mice were challenged by the painting of 1.6% (w/v) 2,4,6-trinitrochlorobenzene in acetone/olive oil (1:1) as an antigen onto the surface of an earlobe. Two hours later, the ear thickness was measured again. Ear edema was calculated according to differences in ear thickness before and after the antigen challenge. To investigate the involvement of IL-10 in the inhibitory effect of PPY, anti-IL-10 antibody or irrelevant IgG (10 µg/100 µL/mouse) adjusted with PBS containing 0.1% BSA was intravenously injected every 2 days during oral administration of the sample. F-fucoidan (200 µg/mouse/day) was orally administered and used as a positive control.
Measurement of IL-10 in serum After the PCA reaction, mice were sacrificed by the collection of blood from the heart under anesthesia by Nembutal injection. Whole blood was left undisturbed for 30 min at room temperature, then centrifuged at 1 200 × g at 4 °C for 15 min. The supernatants were collected as blood sera. Serum samples were stored at -80 °C until analysis. The serum IL-10 concentration was measured by ELISA in accordance with the manufacturer's standard protocol (Life Technologies).
β-Hexosaminidase assay To evaluate the anti-allergy effects of the test samples, an in vitro assay using RBL-2H3 cells was performed in accordance with a previous study (Yamashita et al., 2016). RBL-2H3 cells were seeded at 2.0 × 105 cells/well onto 24-well tissue culture plates in RPMI 1640 and incubated overnight. Then, cells were washed three times with Siraganian buffer (SB; 119 mM NaCl, 5 mM KCl, 0.4 mM MgCl2, 1 mM CaCl2, 40 mM NaOH, 25 mM PIPES, 5.6 mM glucose, 0.1% BSA, pH 7.2) and exposed to test sample solutions for 2 h at 37 °C. Afterward, the RBL-2H3 cells were incubated with anti-DNP IgE at a final concentration of 1 µg/mL for 1 h. After replacing all media with SB, the cells were challenged with 500 µL/well of 100 ng/mL DNP-BSA for 1 h at 37 °C. The plate was cooled in an ice bath for 10 min to stop the degranulation responses. The supernatant (50 µL) was transferred to a 96-well plate, and incubated with an equal volume of substrate solution (2 mM p-nitrophenyl-N-acetyl-β-D-glucosaminide in 0.2 M citrate buffer at pH 4.5) for 1 h at 37 °C. After adding 100 µL/well of stop buffer (0.2 M glycine-NaOH, pH 13.0), the absorbance at 405 nm was measured using a microplate reader. The percentage of β-hexosaminidase released into the supernatants was calculated as a percentage of the degranulation group.
Caco-2/RBL-2H3 cell co-culture system A co-culture system composed of Caco-2 and RBL-2H3 cells was used based on our previous study (Yamashita et al., 2016). Caco-2 cells were seeded at 0.7 × 105 cells/well onto 24-well Transwell insert plates (0.03 cm2, 0.4 µm pore size; Corning Costar Corp., Cambridge, MA, USA). Cell culture medium was changed every 3 days until the cells were fully differentiated (TER value >300 Ω•cm2). RBL-2H3 cells were seeded at 2.0 × 105 cells/well onto 24-well tissue culture plates in RPMI 1640 and incubated overnight. Then, cells were washed three times with SB, and Transwell inserts on which Caco-2 cells had been cultured were added into the plate wells preloaded with RBL-2H3 cells. In addition, 0.2 mL of RPMI 1640 or test sample solution was applied into the apical side. After incubation for 6 h, RBL-2H3 cells were incubated with anti-DNP IgE at a final concentration of 1 µg/mL for 1 h. After replacing all media with SB, RBL-2H3 cells were challenged with DNP-BSA at a final concentration of 100 ng/mL for 1 h at 37 °C. The plate was cooled in an ice bath for 10 min to stop the degranulation responses, and then applied to the β-hexosaminidase assay.
Statistical analysis Each result is presented as the mean ± standard error (SE). Statistical significances among each group were evaluated by analysis of variance (ANOVA), and the Tukey-Kramer test was used to determine differences between groups. Statistical significance was defined as p < 0.05.
The inhibitory effect of PPY on type I hypersensitivity The PCA reaction is a simple method to investigate the inhibitory effects of ingested test samples on type I hypersensitivity; thus, the PCA reaction was performed in mice to evaluate the inhibitory effects of each P. yezoensis f. narawaensis extract in an in vivo experiment. F-fucoidan was used as a positive control, since it possessed inhibitory activity against IgE-mediated ear edema in the PCA reaction (Tanino et al., 2016). Oral administration of PPY and CPY for 4 days significantly inhibited ear edema when compared with the degranulation group (Fig. 1). The inhibition ratio of PPY and CPY was approximately 39% and 27%, respectively. Thus, PPY possessed higher activity than CPY, and was selected for the subsequent experiments. As shown in Fig. 2, PPY significantly decreased ear edema in a dose-dependent manner.

Effects of each fraction from P. yezoensis f. narawaensis on PCA reaction.
Two fractions of P. yezoensis f. narawaensis (1 mg/mouse) and F-fucoidan (200 µg/mouse) were orally administered for 4 days before anti-TNP IgE sensitization. After 30 min, mice were challenged by painting 1.6% (w/v) 2,4,6-trinitrochlorobenzene (Ag) in acetone/olive oil (1:1) onto the surface of an earlobe. Ear edema was calculated according to differences in ear thickness before and after antigen challenge. Values represent means ± SE (n=5). Items with different letter were significantly different (p < 0.05).

Dose dependent manner of PPY in PCA reation.
PPY were orally administered to mice at concentration of 250, 500 and 1 000 mg/mL. PCA reaction was carried out as described in Figure 1. Values represent means ± SE (n = 5). Items with different letter were significantly different (p < 0.05).
Association of IL-10 secretion in the inhibition of type I hypersensitivity by the oral administration of PPY IL-10, an anti-inflammatory cytokine, has been reported to suppress food allergy (Frossard and Eigenmann, 2008). IL-10 suppresses degranulation by decreasing the expression levels of FcεRI on mast cells (Hutchins et al., 2013). It has been demonstrated that the oral administration of polysaccharides from red algae induces the production of IL-10 in serum (Han et al., 2019). As it was hypothesized that PPY might suppress ear edema via IL-10 production, oral administration of PPY for 4 days was tested to confirm whether it could potentiate IL-10 secretion in blood. IL-10 levels in serum were measured by ELISA, and the levels remained almost the same with or without challenge by antigen. However, when PPY was administered orally for 4 days, IL-10 levels increased significantly to about 1.8-fold (Fig. 3).
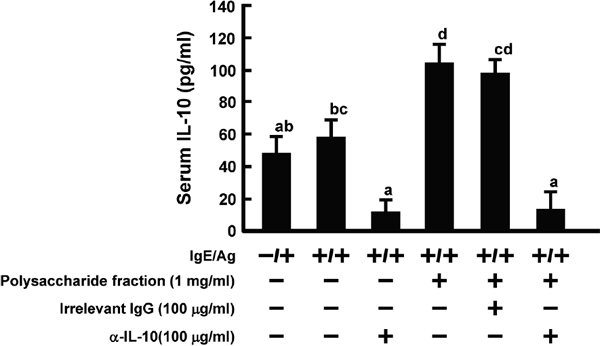
IL-10 concentration of mice administered PPY.
PPY (1 mg/mouse) were orally administered to mice for 4 days, and PCA reaction was performed as described in Materials and Methods. After PCA reaction, mice were sacrificed by collection of blood from heart under anesthesia injected nembutal. Serum IL-10 levels were measured by ELISA. Anti-IL-10 antibody and irrelevant IgG were intravenously injected every two days during oral administration of PPY. Values represent means ± SE (n=5). Items with different letter were significantly different (p < 0.05).
As it was ascertained that PPY can increase IL-10 levels in blood, as was also reported in a previous study (Han et al., 2019), we investigated the association of IL-10 with the inhibition of type I hypersensitivity. To neutralize IL-10, anti-IL-10 antibody was intravenously injected. As shown in Fig. 4, intravenous injection of anti-IL-10 antibody cancelled out the inhibition of ear edema due to PPY administration, but that of irrelevant IgG did not. Irrelevant IgG did not affect the level of IL-10 in serum (Fig. 3). However, serum IL-10 levels diminished to a basal level by the injection of anti-IL-10 antibody, because of the reaction with anti-IL-10 antibody. It was demonstrated that an increase of IL-10 in blood contributed to PPY-induced suppression of type I hypersensitivity.

Effect of anti-IL-10 antibody on PCA reaction.
Values represent means ± SE (n=5). Items with different letter were significantly different (p < 0.05).
Involvement of the intestine in the suppression of type I hypersensitivity by PPY Orally administered PPY possessed suppressive activity against type I hypersensitivity in the PCA reaction (Fig. 1). However, it was not clarified whether the PPY property depended on contact with the intestine. Mice were administered PPY by oral and intraperitoneal injection. Ear edema was suppressed by oral administration of PPY, but not by intraperitoneal injection (Fig. 5), suggesting that intestinal epithelial cells were necessary for PPY to exert its suppression of type I hypersensitivity. Similarly, fucoidan has been reported to suppress type I allergy only by oral administration (Tanino et al., 2016).

Difference in injection method of PPY in PCA reaction.
Mice (n = 5) were orally (p.o.) or intraperitoneally (i.p.) administered PPY (1 mg/day) for 4 days and induced allergic symptoms by PCA reaction. Ear edema was evaluated 2 h after antigen challenge. Values represent means ± SE (n=5). Items with different letter were significantly different (p < 0.05).
Effect of PPY on the degranulation of RBL-2H3 or co-culture system composed of Caco-2 and RBL-2H3 cells It has been reported that IL-10 suppressed FcεRI expression on mast cells, which plays an important role in causing degranulation in allergic responses (Gillespie et al., 2004). Therefore, the influence of PPY on degranulation was investigated using the RBL-2H3 and Coco-2/RBL-2H3 co-culture system. Since the interaction between intestinal epithelial cells and PPY was necessary for type I hypersensitivity suppression (Fig. 5), predictably, β-hexosaminidase release, which is an index of degranulation, showed no drastic change when RBL-2H3 cells were directly treated with PPY at a concentration of 1 mg/mL, a dose which showed no cytotoxicity (Fig. 6). Contrary to expectations, no release of β-hexosaminidase was found in the co-culture system, in which interaction with intestinal epithelial cells can be inferred.

Effect of PPY treatment to RBL-2H3 monolayer and Caco-2/RBL-2H3 co-culture system on β-hexosaminidase release.
PPY was added to RBL-2H3 monolayer directly (A) or apical side of Caco-2/RBL-2H3 co-culture system (B). Luteolin was used as a positive control at concentrations of 100 (A) or 250 (B) µM. The percentage of β-hexosaminidase released into the supernatants was calculated as a percentage of the degranulation group. Values represent means ± SE (n=3). Items with different letter were significantly different (p < 0.05).
Type I hypersensitivity diseases, including hay fever, food allergy, and asthma, are major health problems around the world, and the number of patients suffering from these diseases continues to increase (Galli et al., 2008). Against this background, effective treatments for these diseases have been sought, and in recent years, many studies have focused on food factors. It has been reported that type I allergy is suppressed by many food factors, such as flavonoids (Wu et al., 2006; Kim et al., 2009), polyunsaturated fatty acids (Wang et al., 2015), Lactobacillus species (Murosaki et al., 1998; Shida et al., 2002; Lee et al., 2013), and polysaccharides (Ramberg et al., 2010; Tanino et al., 2016; Mizuno et al., 2020). It has been reported that red alga components suppressed type I hypersensitivity by intraperitoneal administration (Liu et al., 2015; Shi et al., 2015). However, there are few reports on the inhibition of type I hypersensitivity by the oral administration of red algae.
In this study, the color (CPY) and the polysaccharide (PPY) fractions were fractionated from the red alga P. yezoensis f. narawaensis. The oral administration of CPY and PPY fractions showed inhibitory effects on ear edema in the PCA reaction, with inhibition ratios of approximately 27% and 39%, respectively (Fig. 1). Moreover, PPY dose-dependently decreased ear edema (Fig. 2). It has been reported that oral administration of polysaccharides from the red algae Porphyra haitanensis and Gracilaria lemaneiformis induced the production of IL-10 in serum and attenuated OVA-induced anaphylaxis (Han et al., 2019). Therefore, IL-10 levels were measured in the sera of mice administered PPY. Notably, IL-10 levels were increased by PPY administration (Fig. 3). As it was clear that PPY administration enhanced IL-10 levels in blood, the association between IL-10 production and ear edema suppression was investigated using anti-IL-10 antibody, which was intravenously injected before anti-TNP IgE sensitization. As shown in Fig. 4, the ear edema induced by antigen challenge was cancelled out by anti-IL-10 antibody, but not by irrelevant IgG. IL-10 levels in serum did not change in each group of mice injected with anti-IL-10 antibody in the presence or absence of PPY (Fig. 3). These results suggested that the enhancement of IL-10 levels in blood by PPY administration may directly or indirectly suppress type I hypersensitivity. Moreover, the inhibitory effect of PPY on ear edema was shown only when it was administered orally, but not intravenously (Fig. 5). It is likely that the interaction of intestinal epithelial cells with PPY is required for the secretion of IL-10 into the blood.
Mast cells are key effector cells on which IgE cross-links with FcεRI. The cross-linking of food allergens with IgE-FcεRI further triggers the production and release of anaphylactic mediators, such as histamine and β-hexosaminidase, which results in allergic reactions (Liu et al., 2018; Moñino-Romero et al., 2019). On the other hand, there is a report that IL-10 suppresses FcεRI expression on mast cells (Gillespie et al., 2004). The effect of PPY on the degranulation of mast cells was investigated using RBL-2H3 and a co-culture system composed of Caco-2/RBL-2H3 cells; no effect was seen on β-hexosaminidase release in either situation, suggesting that mast cell degranulation was not directly inhibited by PPY or through intestinal epithelial cells (Fig. 6). As the PCA reaction was suppressed only by the oral administration of PPY (Fig. 5), it made sense that direct treatment of RBL-2H3 cells with PPY did not suppress degranulation. However, it was unexpected that β-hexosaminidase release was not inhibited when PPY was added into the apical side of the co-culture system, which can reflect the interaction with intestinal epithelial cells. These results suggested that PPY has to be recognized by intestinal epithelial cells to stimulate IL-10 production, but it does not directly regulate the degranulation of mast cells. Hence, the inhibitory effect of PPY on the PCA reaction is due to some other mechanism.
The molecular mechanism underlying how PPY enhanced IL-10 production is still not well understood in this study. However, IL-10 is a representative immunosuppressive cytokine. IL-10 is known to suppress the production of IL-4 and IL-5 by inhibiting the activity of Th2 cells (Borish, 1999). Moreover, IL-10 suppresses B cell differentiation and antibody production (Moore et al., 2001). Taken together, these reports suggest that IL-10 plays an important role in the treatment of allergy. It was demonstrated that a polysaccharide from Aloe vera suppressed Th2 immune responses by stimulating the secretion of IL-10 in food allergy mice, and increased the population of IL-10-producing type 1 regulatory cells (Lee et al., 2018). However, since PPY exerted anti-allergic activity only by oral administration, and not intraperitoneal administration, it seems unlikely that PPY could directly stimulate IL-10-producing type 1 regulatory cells. Further studies to determine this mechanism will help illuminate the different down-modulating effects of IL-10 on anti-allergy. In conclusion, this study is the first in vivo study in which PPY was shown to ameliorate type I hypersensitivity with IL-10 secretion into blood. Our research suggested that polysaccharides prepared from P. yezoensis f. narawaensis might provide a novel inhibitory or therapeutic food factor for allergy diseases.
antigen presenting cells
CMCcarboxymethyl cellulose
CPYcolor fraction from P. yezoensis f. narawaensis
IL-10interleukin 10
PPYpolysaccharide fraction from P. yezoensis f. narawaensis
IgEimmunoglobulin E
DNP IgEdinitrophenyl IgE