2021 Volume 27 Issue 1 Pages 43-48
2021 Volume 27 Issue 1 Pages 43-48
The phase behavior of a mixture of rapeseed and soybean oils during cooling and heating was observed through differential scanning colorimetry (DSC). The mixture was cooled from 50 to −80 °C at 4 °C/min and heated from −80 to 50 °C at 2 °C/min. A polymorphic transition was observed during heating when the mixture contained soybean oil at a weight fraction of ≥ 0.7. Comparing the results presented herein with those for a binary mixture of high-purity triacylglycerols (TAGs) indicated that some peaks could be ascribed to the formation and melting of crystals rich in triolein and trilinolein. The total released or absorbed energies during cooling or heating were calculated from the DSC curves and were affected by the polymorphic transition and TAG interactions.
Lipids constitute essential nutrients for humans and are ingested through foods. A common source of ingested lipids is vegetable oil derived from plant seeds and is composed mainly of triacylglycerol (TAG). Vegetable oils are typically used for cooking and seasoning (Nakanishi, 2014) and as raw materials for manufactured food products. Understanding the phase behavior of TAG is important for efficient vegetable oil refining (Totani and Hara, 2015; Gordon and Rahman, 1991), production of high-quality foods containing vegetable oil (Campos et al., 2002; Lipp and Anklam, 1998), and preservation of food quality (Coupland, 2002; Rousseau, 2000). In terms of the phase behavior of TAG, polymorphisms have been extensively studied through X-ray diffraction and differential scanning calorimetry (DSC) (Bayés-García et al., 2017; Rincón-Cardona et al., 2013; Himawan et al., 2007; MacNaughtan et al., 2006). Polymorphism, wherein the molecules are packed in different crystalline arrangements, occurs when long-chain molecules crystallize (Himawan et al., 2006; Sato, 2001). Polymorphs of fats are classified, in many cases, into three forms, α, β′, and β (Larsson, 1966), which are thermodynamically as stable as the latter. Crystallization behavior, crystal size, crystal morphology, and physical properties differ depending on the polymorphic form, and transition to a stable form may be induced by external factors including temperature changes (Sato, 2001). Recently, crystal size and morphology have been studied by polarized microscopy (Domingues et al., 2018; Campos et al., 2002) along with mechanical hardness (Domingues et al., 2018; Daels et al., 2018; Campos et al, 2002), solid fat content using pulsed nuclear magnetic resonance (Daels et al., 2018; Nusantoro et al., 2017; Bootello et al., 2013), and solid fat index by dilatometry (Miyagawa et al., 2019, 2018; Yoshida et al., 2019).
Previously investigated oils include coconut oil (Gordon and Rahman, 1991), cocoa butter (da Silva et al., 2017; Lipp and Anklam, 1998), palm oil (Daels et al., 2018; Domingues et al., 2018), palm kernel oil (Nusantoro et al., 2017), sunflower oil (Bootello et al., 2013; Rincón-Cardona et al., 2013), and olive oil (Bayés-García et al., 2017; Barba et al., 2013; Chiavaro et al., 2012) as well as high-purity TAGs (Himawan et al., 2007; MacNaughtan et al., 2006; Sato, 2001). The phase behavior of vegetable oils containing monoacylglycerols and various additives has also been studied (Daels et al., 2018; Domingues et al., 2018; Smith et al., 2011; Gordon and Rahman, 1991). Vegetable oils and TAGs that have been studied to date contain significant amounts of high melting point fatty acids including lauric, palmitic, and stearic acids, which usually crystallize at room temperature or above. The phase behavior of vegetable oils, including sunflower and olive oils, that crystallize between room temperature and commercial refrigeration temperature, has also been studied. However, the behavior of vegetable oils with a lower melting point that crystallize below commercial refrigeration temperatures has not been thoroughly investigated.
We have investigated the crystallization behavior of vegetable oils with low melting points, including rapeseed and soybean oils, using DSC and dilatometry (Miyagawa and Adachi, 2019; Miyagawa et al., 2020, 2018). Rapeseed and soybean oils are commonly used as raw materials for edible oils, and are typical vegetable oils rich in triolein and trilinolein. The components of these vegetable oils were examined along with the phase behavior of a binary mixture of high-purity triolein and trilinolein, as well as that of mixtures of these TAGs and tripalmitin (Nagamizu et al., 2020). In order to deepen our understanding of the crystallization and melting behavior of mixed rapeseed and soybean oils, thermal changes were determined using DSC to further understand the phase behavior of vegetable oils with low melting points.
Materials Rapeseed and soybean oils (first grade) were purchased from Fujifilm Wako Pure Chemical (Osaka, Japan).
DSC curves Samples were prepared by mixing rapeseed and soybean oils at various weight fractions. Samples (6 to 12 mg) were accurately weighed into an aluminum seal cell (Al6f1.5, Shimadzu, Kyoto, Japan) and the DSC curve was measured using a DSC-50 (Shimadzu). Two measurements were performed on samples with similar weight fractions of soybean oil in the mixture with an empty cell as a reference. After the mixture was maintained at 50 °C for 10 min, it was cooled to −80 °C at 4 °C/min with liquid nitrogen using an LTO server (LTS-50, Shimadzu). Immediately after reaching −80 °C, the mixture was heated to 50 °C at 2 °C/min. The total energies released and absorbed during cooling and heating in the temperature range of 30 to −80 °C were calculated from the DSC curves. During cooling, the slope of the baseline changed and was corrected using a cubic curve passing through the values at 30, 20, 10, 0, −75, and −80 °C. During heating, no remarkable heat release or heat absorption was observed from −80 to −55 °C, and the baseline was unstable. Therefore, the absorbed energy in this temperature range was set to 0. Because the slope of the baseline changed slightly and monotonically during heating, the values at −50, 5, 10, 20, and 30 °C were linearly approximated to correct the baseline. The values used for the baseline correction fell within a range that did not contain any obvious exothermic or endothermic peaks. The total released or absorbed energy was evaluated from the area of the DSC curve and baseline (value at which the energy was set to 0) by considering the cooling or heating rate. During cooling and heating, heat release and absorption are expressed as positive values.
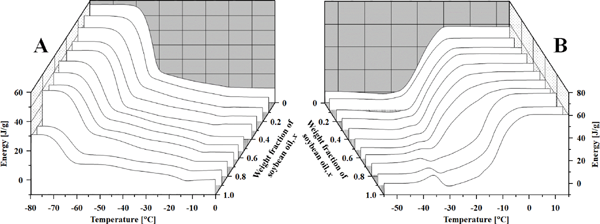
Thermal changes during cooling and heating Figure 1 shows representative baseline-corrected DSC curves of the rapeseed and soybean oil mixtures during cooling and heating. The temperature range which showed remarkable thermal changes was 0 to −80 °C for cooling and −55 to 15 °C for heating. As mentioned above, heat release and absorption are expressed as positive for cooling and heating. Figure 2 shows the peak-top temperatures of the peaks observed for the various weight fractions of soybean oil. During cooling, the slope of the baseline changed sequentially from the start of cooling (50 °C) to the first thermal change, with thermal changes observed from −18 to −10 °C (weight fraction of soybean oil, x = 0 to 0.9), from −20 to −15 °C (at all x values), from −30 to −23 °C (x = 0.2 to 0.9), from −43 to −40 °C (at all x values), and from −66 to −53 °C (at all x values). Only the peak from −18 to −10 °C was endothermic, while the others were exothermic. The change in the slope of the baseline suggested a change in the specific heat of the sample. This indicated a change in the mobility of the TAG molecules and interactions between them. It is speculated that the mobility of TAG molecules decreased and/or became more ordered during cooling. The endotherm observed thereafter suggested that this change was moderate prior to crystallization. Therefore, subsequent crystallization may proceed via a melt-mediated transition (Himawan et al., 2006; Sato, 2001).

DSC curves, which were baseline corrected, in (A) cooling and (B) heating.

Peak top temperatures of the DSC curves in (A) cooling and (B) heating. (A): ●;−18 to −10 °C, ○; −20 to −15 °C, △; −30 to −23 °C, □; −43 to −40 °C, ◇; −66 to −53 °C. (B): ●; −3 to −6 °C, ▲; −9 to −15 °C, ■; −17 to −19 °C, ◆; −19 to −23 °C, ◀; −23 to −30 °C, ▼; −29 to −40 °C, ○; −32 to −36 °C, ▶; −39 to −48 °C. Open and closed symbols represent exothermic and endothermic peaks, respectively. The solid lines indicate the peak top temperatures, and the broken lines indicate the top temperatures of the shoulder peaks.
The peaks observed during heating can be classified as those belonging to one of the three types. The symbols connected by the broken curves in Fig. 2B indicate the peak temperatures of the shoulder peaks. The first peak type includes the three endothermic peaks observed from −19 to −23 °C (at all x values), −23 to −30 °C (x = 0.1 to 1), and −29 to −40 °C (at all x values), the temperatures of which decreased linearly with increasing x. The second type includes the two endothermic peaks observed from −3 to −6 °C and −9 to −15 °C, with the peak temperature and shape changing at x = 0.5. In a binary system, such as SOS/OSO, SOS/SSO, POP/OPO, and POP/PPO, where O, P, and S represent oleic, palmitic, and stearic acids, respectively, a novel molecular structure was formed for TAGs mixed in equal amounts (Sato, 2001), and the crystallization rate changed in mixture with nearly equal components (Himawan et al., 2007; MacNaughtan et al., 2006). Some TAGs are found only in rapeseed or soybean oil (Murui and Shouge, 1996), suggesting that the change in the peak from −10 to −15 °C at x = 0.5 could be ascribed to the formation of molecular compounds through the interaction of TAGs contained in either oil.
The third type consists of the three peaks that existed or not near x = 0.6 to 0.7. An endothermic peak at −39 to −48 °C was observed in the range of x = 0 to 0.6. An exothermic peak from −32 to −36 °C and an endothermic peak from −17 to −19 °C were observed at x = 0.7 to 1. The peak-top temperature of the latter peak increased with increasing x. Because the peaks observed at x = 0.7 to 1 can be categorized as crystallization and melting, they were attributed to the formation of a more stable crystal (polymorphic transition) and crystal melting, respectively. The phase behavior of a mixture of high-purity triolein (OOO) and trilinolein (LLL) was previously investigated (Nagamizu et al., 2020). A polymorphic transition was observed during heating at LLL weight fractions of 0.6 to 1, and the melting point of the crystal after the transition was −14 to −12 °C, which increased with increasing LLL weight fraction. The crystal appeared to be an LLL-rich crystal consisting of OOO and LLL (Nagamizu et al., 2020). Although the fatty acid and TAG compositions of vegetable oils depend on the harvest location and time of the raw materials, the content of oleic acid in rapeseed and soybean oils has been reported to be approximately 65% and 25%, and that of linoleic acid to be approximately 20% and 53%, respectively (Miyagawa et al., 2019). For TAG compositions, the OOO content in rapeseed and soybean oils has been reported to be 24% and 3%, respectively, with LLL contents of 1% and 18%, respectively, (Murui and Shouge, 1996). Based on these compositions, the above-mentioned value where the peak varies corresponds to x = 0.6 and 0.7. The melting temperature (−19 to −17 °C) observed herein was lower than that for the mixture of high-purity TAGs. However, the temperature increase trend as a function of x remained constant. Minor components can disrupt the dense packing of molecules, causing imperfections in the crystal structure (Himawan et al., 2006) and lowering the crystal melting point. The small amount of TAG present in the system acts as an endogenous minor component and affects crystallization behavior (Smith et al., 2011). Many TAGs other than OOO and LLL are included in the system studied herein. Therefore, the peaks observed at x = 0.7 to 1 likely originated from the formation and melting of LLL-rich crystals composed of OOO and LLL as well as some other minor TAGs, which are similar in structure but lower in content to OOO and LLL and crystalize together with OOO and LLL (Miyagawa and Adachi, 2019). The peaks observed from x = 0 to 0.6 were ascribed to the melting of OOO-rich crystals of OOO and LLL containing other TAGs as well.
Release and absorbed energies during cooling and heating Figure 3 shows representative examples of cumulative released or absorbed energies during cooling or heating obtained by integrating the DSC curves. Figure 4 shows the dependence of the total released and absorbed energies on the weight fraction of soybean oil. The total released energy decreased with increasing x. Because the polymorphic transition was more significant with increasing x, the crystals seemed to destabilize and/or the fraction of unstable crystals increased. The melting energies of the β crystals of OOO and LLL are approximately 108 and 96 J/g, respectively (Hagemann and Tallent, 1972). As soybean oil contains more LLL than rapeseed oil, it exhibits a lower crystallization energy even in the same crystal form. The lower total crystallization energy of soybean oil reflects both the polymorphic transition and fatty acid composition. In contrast, the total absorbed energy increased with increasing x.
The total energies (A) released during cooling and (B) absorbed during heating.

The total released energy in cooling (closed symbols) and the total absorbed energy in heating (open symbols) for the mixtures with various weight fractions of soybean oil. Inset: The relationship of the relative energy, defined as the ratio of the absorbed energy to the released energy, and the weight fraction.
The absorbed energy includes the energy required to melt both crystals produced during cooling and the stable crystal via polymorphic transition and for the formation of the stable crystal. Although the latter energy negated some of the absorbed energy, the overall effect was insignificant because the energy was significantly lower than that required for the solid-liquid transition, that is, the melting energy of the stable crystal. Therefore, the total absorbed energy increases with increasing proportion of stable crystals via polymorphic transition. In addition, the released and absorbed energies for the mixtures containing small amounts of the other oil (x = 0.1 and 0.9) were slightly lower than those of pure rapeseed or soybean oil during cooling and heating. As mentioned above, minor components reduce crystal stability (Himawan et al., 2006). It is likely that when either oil was present below 10%, the TAGs contained only in the other oil disrupted dense packing as an impurity. In contrast, near x = 0.5, the total energy was almost the same as that of either pure rapeseed or soybean oil. Therefore, it is possible that TAGs contained only in a single oil interacted with each other to form a molecular structure near x = 0.5. Thus, the total energy may not be reduced significantly. The relative energy, defined as the ratio of the total absorbed energy to the released energy, increased significantly at x ≥ 0.7 (inset of Fig. 4). This was in agreement with the DSC curves during heating, which showed a clear polymorphic transition at x ≥ 0.7. These results also suggest that the increased crystal stability due to polymorphic transition may be the underlying mechanism for the increased total energies.
The crystallization and melting behaviors of the mixtures of rapeseed and soybean oils were investigated through DSC. During cooling, an endotherm, or melting, was confirmed immediately before crystallization, suggesting that the previously formed state and/or structure moderates and crystallizes. During heating, polymorphic transition was observed at a soybean oil weight fraction of x ≥ 0.7. Comparison of the results presented herein with the crystallization and melting behaviors of a mixture of high-purity triolein and trilinolein suggested that some exothermic and endothermic peaks were related to the formation and melting of crystals containing triolein and trilinolein. Even the phase behavior of vegetable oil mixtures containing more than a dozen triacylglycerols can be interpreted based on the results of a binary mixture of high-purity TAGs.
The total released and absorbed energies during cooling and heating were also calculated. From the relationship between the total energies and weight fraction of soybean oil, the interaction between TAGs changed as a function of soybean oil content. The ratio of the absorbed to released energy increased remarkably at x ≥ 0.7, suggesting the influence of a polymorphic transition.